Global Prosthetic Joint Infection Treatment Market - Key Trends & Drivers Summarized
Why Are Prosthetic Joint Infections Emerging as a Major Orthopedic Complication Requiring Specialized Treatment Pathways?
Prosthetic joint infections (PJIs) are among the most serious and costly complications following joint arthroplasty procedures. While the global volume of total hip and knee replacements continues to rise due to aging populations and lifestyle-related joint degeneration, infection rates-though low-pose a high clinical and economic burden. PJIs typically occur due to microbial colonization of the implant surface, forming biofilms that are resistant to immune response and conventional antibiotics. These infections necessitate complex, prolonged treatment regimens involving surgery, antimicrobial therapy, and long-term rehabilitation.Early-onset PJIs (within three months post-surgery) often involve virulent organisms such as Staphylococcus aureus, while delayed or late infections are frequently caused by coagulase-negative staphylococci and other low-grade pathogens. These distinctions influence treatment decisions, which may range from debridement with implant retention (DAIR) to two-stage revision arthroplasty, depending on infection chronicity and patient health. As surgical volumes increase globally-particularly in Asia-Pacific and Europe-the need for standardized, evidence-based PJI treatment protocols is becoming increasingly urgent.
Which Surgical and Pharmaceutical Approaches Are Being Utilized to Manage PJIs Across Different Patient Profiles?
The cornerstone of PJI treatment is surgical intervention combined with targeted antimicrobial therapy. In acute infections diagnosed within a short post-operative window, DAIR is considered if the implant remains stable and the pathogen is known. However, in chronic or treatment-resistant infections, explantation followed by a two-stage revision remains the gold standard, with antibiotic-loaded spacers used during the interim. One-stage revisions are gaining ground in select cases, particularly in Europe, due to reduced morbidity and healthcare cost savings.Antibiotic stewardship is crucial given the high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and biofilm-forming bacteria. Empirical therapy typically begins with broad-spectrum agents such as vancomycin or daptomycin, followed by de-escalation based on culture sensitivity. Local antibiotic delivery via cement spacers, beads, or coatings enhances drug concentration at the infection site while minimizing systemic toxicity. Long-term oral suppressive antibiotics may be used in patients who are not candidates for surgical revision. Personalized treatment plans are shaped by pathogen type, biofilm maturity, patient immune status, and comorbidity burden.
How Are Diagnostic Tools, Infection Control Policies, and Hospital Infrastructure Influencing Treatment Outcomes?
Accurate diagnosis of PJIs remains challenging, often requiring a combination of serum markers (e.g., CRP, ESR), synovial fluid analysis, imaging, and microbiological culture. Advanced diagnostic modalities such as next-generation sequencing (NGS), polymerase chain reaction (PCR), and alpha-defensin immunoassays are improving pathogen detection, especially in culture-negative cases. Imaging techniques such as PET-CT and labeled leukocyte scintigraphy are aiding in identifying occult infections and prosthesis loosening. However, these tools are not yet widely available in low-resource settings.Infection prevention remains the most effective strategy, with strict operating room protocols, laminar airflow systems, and preoperative screening for nasal S. aureus colonization now standard in many centers. Some hospitals are integrating multidisciplinary PJI teams involving orthopedic surgeons, infectious disease specialists, and clinical microbiologists to ensure timely diagnosis and coordinated care. Institutional registries and national joint replacement databases are playing a growing role in monitoring infection rates, tracking revision outcomes, and refining best practices.
What Factors Are Driving the Growth of the Global Prosthetic Joint Infection Treatment Market?
The growth in the global prosthetic joint infection treatment market is driven by rising arthroplasty volumes, increasing patient life expectancy, and enhanced infection surveillance. As the global burden of osteoarthritis and rheumatoid arthritis grows, the number of total joint replacements is expected to rise steadily-leading to a parallel increase in PJI incidence. Additionally, older adults with comorbidities such as diabetes, obesity, and immunosuppression are at higher risk for infection, necessitating more robust treatment options.Pharmaceutical innovation is also playing a role, with new antibiotic formulations, biofilm-targeting agents, and bacteriophage therapies under investigation. Growth in orthopedic biomaterials with antimicrobial properties-such as silver-coated implants, iodine-impregnated prosthetics, and antimicrobial hydrogel coatings-holds promise for infection prevention. Hospitals are investing in dedicated infection control units, revision surgery suites, and long-term care support to manage the clinical and financial complexities of PJIs.
Leading players in this market include Zimmer Biomet, Stryker, DePuy Synthes (Johnson & Johnson), Heraeus Medical, and Biocomposites. These companies are enhancing their offerings in antibiotic-loaded cement, revision implants, and diagnostic kits. As clinical guidelines continue to evolve and data on treatment outcomes accumulate, the prosthetic joint infection treatment market is expected to grow in both sophistication and scale-shifting toward multidisciplinary, patient-specific, and cost-efficient care models.
Scope Of Study:
The report analyzes the Prosthetic Joint infection Treatment market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Drug (Aminoglycosides, Glycopeptides, Rifamycin, Lincosamide, Penicillin, Other Drugs); Infection (Pre-operative Infection, Post-operative Infection); Administration Route (Oral Administration, Intravenous Administration); Pathogen (Staphylococcus Aureus, Coagulase-negative Staphylococcus, Candida Species, Enterococcus Species, Other Pathogens); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Drug Stores Distribution Channel, Online Distribution Channel)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Aminoglycosides segment, which is expected to reach US$29.2 Million by 2030 with a CAGR of a 2.5%. The Glycopeptides segment is also set to grow at 4.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $29.2 Million in 2024, and China, forecasted to grow at an impressive 6.6% CAGR to reach $26.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Prosthetic Joint infection Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Prosthetic Joint infection Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Prosthetic Joint infection Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aurobindo Pharma Ltd., AstraZeneca PLC, Cipla Ltd., Dr. Reddy’s Laboratories, Eli Lilly and Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this Prosthetic Joint infection Treatment market report include:
- Aurobindo Pharma Ltd.
- AstraZeneca PLC
- Cipla Ltd.
- Dr. Reddy’s Laboratories
- Eli Lilly and Company
- GSK plc
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Pfizer Inc.
- Sanofi
- Smith & Nephew plc
- Stryker Corporation
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- TenNor Therapeutics Ltd.
- Zimmer Biomet
- DePuy?Synthes (J&J)
- 3M Healthcare
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aurobindo Pharma Ltd.
- AstraZeneca PLC
- Cipla Ltd.
- Dr. Reddy’s Laboratories
- Eli Lilly and Company
- GSK plc
- Lupin Limited
- Merck & Co., Inc.
- Mylan N.V.
- Novartis AG
- Pfizer Inc.
- Sanofi
- Smith & Nephew plc
- Stryker Corporation
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- TenNor Therapeutics Ltd.
- Zimmer Biomet
- DePuy?Synthes (J&J)
- 3M Healthcare
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 576 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
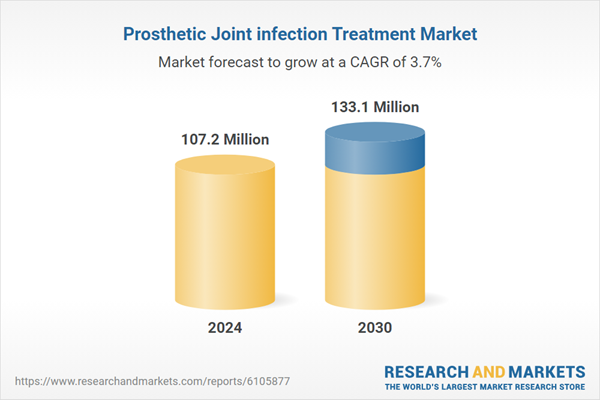
| Estimated Market Value in 2024 | 107.2 Million |
| Forecasted Market Value by 2030 | 133.1 Million |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |