Global Skull Clamps Market - Key Trends & Drivers Summarized
Why Are Skull Clamps Critical in Enhancing Surgical Precision and Operative Safety?
Skull clamps, also known as cranial fixation devices, are specialized neurosurgical tools designed to immobilize the patient-s head during delicate surgical procedures such as craniotomies, spinal operations, or stereotactic interventions. By providing a rigid, three-point fixation of the skull, these devices eliminate unwanted movement, allowing surgeons to operate with exceptional precision in high-risk anatomical zones. The use of skull clamps is indispensable in cranial and cervical spine surgeries, where even millimeter-scale instability can lead to procedural failure or neurological damage. The accuracy required in neuronavigation, endoscopic approaches, and minimally invasive neurosurgical techniques has reinforced the role of skull clamps as standard operating room instrumentation.These devices are designed with torque-limiting screws, radiolucent materials, and ergonomic base attachments that allow for integration with surgical tables and imaging equipment. Pediatric and adult variants, along with adjustable configurations for different head sizes and pathologies, make skull clamps adaptable across surgical specialties and patient demographics. Their application extends beyond neurosurgery into otolaryngology, maxillofacial procedures, and even veterinary neurosurgical practice. The increasing complexity and precision of modern surgical interventions are amplifying the demand for advanced cranial stabilization systems, positioning skull clamps as a foundational component in operative neurosurgical care.
How Are Technological Advancements Enhancing Skull Clamp Performance and Safety?
Recent technological innovations in materials science, biomechanics, and surgical ergonomics are reshaping the design and utility of skull clamps. High-performance radiolucent materials such as carbon fiber-reinforced polymers are replacing traditional metal frames in newer devices, allowing unobstructed intraoperative imaging through CT, MRI, and fluoroscopy. These radiotransparent clamps reduce imaging artifacts and facilitate real-time guidance during tumor resection, aneurysm clipping, or electrode placement procedures. Simultaneously, manufacturers are focusing on weight reduction and improved force distribution across fixation pins to minimize the risk of skull penetration, especially in elderly, osteoporotic, or pediatric patients.Torque-limiting technology has seen significant refinement, with built-in mechanisms that prevent over-tightening and distribute clamping forces more uniformly. Some advanced systems feature audible click-stops and automatic locking to enhance user feedback and prevent user error. Moreover, modular skull clamp systems are being developed with interchangeable parts for anterior-posterior and lateral fixation, giving neurosurgeons greater flexibility based on surgical access needs. Several products now include sterile, disposable pin options to eliminate infection risks and streamline surgical turnover.
In tandem with clamp design, integration with digital operating rooms is becoming standard. Skull clamps are now engineered to interface with neuronavigation systems, robotic arms, and intraoperative imaging gantries. Sensor-embedded clamps with pressure monitoring capabilities are under development to alert surgical staff in real-time to excessive pin force or slippage. These advances are making skull clamps smarter, safer, and more aligned with the needs of precision-guided surgery in modern neurosurgical theaters.
Which Healthcare Settings and Surgical Applications Are Expanding the Demand Base?
Demand for skull clamps is highest in tertiary hospitals, neurosurgical centers, trauma units, and academic medical institutions equipped for complex cranial and spinal procedures. The rise in intracranial surgeries related to brain tumors, traumatic brain injuries (TBI), cerebrovascular disorders, and neurodegenerative diseases has significantly widened the clinical use of cranial fixation systems. Procedures such as deep brain stimulation (DBS), transnasal endoscopic surgeries, and cervical spine decompressions require high-stability head immobilization-reinforcing the clinical reliance on skull clamps.Pediatric neurosurgery is an emerging area influencing product segmentation. Devices specifically designed for infants and children must address skull pliability, fontanelle preservation, and size variability while still offering adequate rigidity. Manufacturers are responding by producing dedicated pediatric skull clamp kits with safety-pin depth indicators and lightweight materials. In veterinary neurosurgery, particularly for small animals, miniaturized skull clamps are used in animal model research and specialized surgical clinics, adding another layer to the niche demand.
Furthermore, as outpatient neurosurgical procedures and same-day surgeries increase, ambulatory surgical centers (ASCs) are investing in lightweight, portable skull clamp systems that offer fast setup, easy disinfection, and ergonomic compatibility with compact surgical tables. Emerging markets in Latin America, Southeast Asia, and the Middle East are also showing rising procurement activity as neurosurgical capacity expands and healthcare infrastructure matures. Global suppliers are targeting these regions through distributor partnerships, local regulatory compliance, and training support.
What Are the Key Drivers Fueling Growth in the Skull Clamps Market?
The growth in the global skull clamps market is driven by several interrelated factors reflecting advances in surgical practice, patient demographics, and device innovation. First and foremost is the global rise in neurosurgical procedures prompted by increasing incidence of traumatic injuries, brain tumors, epilepsy, and spinal disorders. With aging populations and expanding access to diagnostic imaging, more patients are being referred for surgical intervention, thereby increasing the need for precise cranial fixation tools like skull clamps.Second, the integration of navigation-assisted and robotic neurosurgical systems is elevating demand for compatible, precision-engineered skull clamps. These procedures demand sub-millimeter stability, making high-end clamps essential to avoid misalignment and enhance surgical safety. Third, the continuous advancement in biomaterials and manufacturing techniques has made it possible to produce clamps that are not only stronger and lighter but also more customizable, improving surgeon satisfaction and patient outcomes.
Additionally, regulatory bodies and clinical guidelines are emphasizing the importance of safe cranial fixation practices, further embedding skull clamps into standard operating room protocols. The growing trend toward minimally invasive skull base surgeries and stereotactic interventions is accelerating the adoption of adaptable, multi-angle fixation platforms. Training programs and simulation labs across neurosurgical education centers are also contributing to sustained market growth by standardizing clamp usage and brand loyalty from early-career surgeons.
Finally, the increasing availability of disposable clamp components and service contracts is creating recurring revenue streams for device manufacturers. As neurosurgical infrastructure expands globally, especially in emerging healthcare systems, the skull clamps market is set for robust, steady growth supported by surgical innovation, material breakthroughs, and a widening clinical footprint.
Scope Of Study:
The report analyzes the Skull Clamps market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Product (Three-Pin Skull Clamp, Four-Pin Skull Clamp, Two-Pin Skull Clamp); Material (Stainless Steel Material, Aluminum Alloy Material, Titanium Material, Radiolucent Material); Application (Surgery Application, Medical Imaging Application)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Three-Pin Skull Clamp segment, which is expected to reach US$2.0 Billion by 2030 with a CAGR of a 6.1%. The Four-Pin Skull Clamp segment is also set to grow at 9.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $607.5 Million in 2024, and China, forecasted to grow at an impressive 11.5% CAGR to reach $718.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Skull Clamps Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Skull Clamps Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Skull Clamps Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aesculap (B. Braun Melsungen AG), Allen Medical Systems, Inc., Black Forest Medical Group, Changzhou Huida Medical Equipment Co., Ltd., Elekta AB and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Skull Clamps market report include:
- Aesculap (B. Braun Melsungen AG)
- Allen Medical Systems, Inc.
- Black Forest Medical Group
- Changzhou Huida Medical Equipment Co., Ltd.
- Elekta AB
- Eschmann Technologies Ltd.
- IMRIS (Deerfield Imaging, Inc.)
- Integra LifeSciences Corporation
- Johnson & Johnson (DePuy Synthes)
- KLS Martin Group
- Medifa GmbH & Co. KG
- Medtronic plc
- Micromar Indústria e Comércio Ltda.
- Mizuho Medical Co., Ltd.
- Ningbo Cibei Medical Treatment Appliance Co., Ltd.
- OsteoMed LLC
- OPT SurgiSystems S.r.l.
- PMI - Pro Med Instruments GmbH
- Schaerer Medical AG
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aesculap (B. Braun Melsungen AG)
- Allen Medical Systems, Inc.
- Black Forest Medical Group
- Changzhou Huida Medical Equipment Co., Ltd.
- Elekta AB
- Eschmann Technologies Ltd.
- IMRIS (Deerfield Imaging, Inc.)
- Integra LifeSciences Corporation
- Johnson & Johnson (DePuy Synthes)
- KLS Martin Group
- Medifa GmbH & Co. KG
- Medtronic plc
- Micromar Indústria e Comércio Ltda.
- Mizuho Medical Co., Ltd.
- Ningbo Cibei Medical Treatment Appliance Co., Ltd.
- OsteoMed LLC
- OPT SurgiSystems S.r.l.
- PMI – Pro Med Instruments GmbH
- Schaerer Medical AG
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 365 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
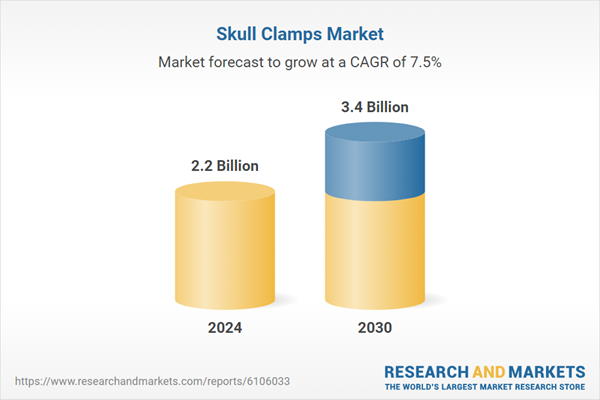
| Estimated Market Value in 2024 | 2.2 Billion |
| Forecasted Market Value by 2030 | 3.4 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |