Global Metered Dose Inhalers Market - Key Trends & Drivers Summarized
How Are Technological Advancements Revolutionizing Metered Dose Inhalers?
Metered Dose Inhalers (MDIs) have long been a cornerstone in the management of respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD), and recent technological innovations are significantly elevating their performance and usability. Historically, MDIs have relied on propellant-based systems to deliver a specific dose of medication directly to the lungs, but current advancements are making these devices more precise, user-friendly, and environmentally sustainable. Digital MDIs equipped with sensors and connectivity features are emerging as a major breakthrough, allowing for dose tracking, adherence monitoring, and real-time feedback via smartphone apps. These smart inhalers are proving valuable not just for patients, but also for clinicians who can monitor inhalation patterns and medication adherence remotely. Additionally, advancements in valve design and particle engineering have improved dose uniformity and lung deposition efficiency, ensuring that patients receive a more consistent therapeutic effect. Some devices now incorporate dose counters and breath-actuated mechanisms, which help eliminate common usage errors such as incorrect timing between actuation and inhalation. Moreover, new formulations are being developed to increase drug stability and compatibility with environmentally friendly propellants. The transition from chlorofluorocarbon (CFC) to hydrofluoroalkane (HFA) propellants is being followed by a push toward even lower global warming potential (GWP) alternatives, driven by regulatory and corporate sustainability goals. Innovations in canister coatings and suspension formulations are also helping extend shelf life and minimize drug loss. Together, these advancements are driving the evolution of MDIs from traditional delivery tools into sophisticated therapeutic devices tailored for modern, connected healthcare ecosystems.What Role Do Regulatory Policies and Environmental Concerns Play in Market Dynamics?
Regulatory oversight and growing environmental awareness are exerting substantial influence on the metered dose inhalers market, compelling manufacturers to adapt both their product design and production strategies. The global shift toward reducing greenhouse gas emissions has brought MDIs under scrutiny, primarily due to their reliance on HFA propellants that contribute to climate change. Regulatory bodies in regions such as the European Union and North America are increasingly pushing for eco-friendlier alternatives, prompting pharmaceutical companies to invest in low-GWP or non-propellant technologies. This has led to accelerated research into sustainable propellants like hydrofluoroolefins (HFOs) and even the consideration of dry powder inhalers (DPIs) as greener alternatives in some treatment scenarios. Health authorities are also tightening regulations on product labeling, dose accuracy, and child-resistance features, which is influencing design considerations for future MDI models. Moreover, obtaining regulatory approval for reformulated inhalers with new propellants or delivery mechanisms requires extensive clinical testing and documentation, which can delay market entry and increase development costs. At the same time, countries are introducing policies to ensure broader access to respiratory therapies, which is encouraging generic drug manufacturers to enter the MDI space with more affordable options. This increased competition is pressuring established players to balance innovation with cost-efficiency. Additionally, pharmacovigilance mandates are becoming stricter, requiring ongoing monitoring of post-market performance and adverse events. As a result, regulatory compliance is now not just a barrier to entry but also a continuous operational priority. Manufacturers that can navigate this dual challenge of environmental responsibility and regulatory stringency are more likely to sustain long-term growth and market relevance.How Are Shifting Patient Needs and Healthcare Models Impacting MDI Design?
Patient expectations and the transformation of global healthcare delivery models are playing a critical role in shaping the evolution of metered dose inhalers. Today's patients are more informed and actively engaged in managing their chronic conditions, prompting a demand for inhalers that offer greater ease of use, reliability, and feedback mechanisms. One of the persistent challenges with MDIs has been the correct coordination of actuation and inhalation, especially among elderly populations and children. To address this, manufacturers are increasingly developing breath-actuated MDIs and adding visual or auditory cues that guide proper usage. Moreover, as healthcare systems move toward telemedicine and home-based care, devices that can transmit usage data remotely are gaining traction. This is particularly important for chronic disease management, where regular monitoring and timely adjustments to therapy are essential. The growing prevalence of comorbidities is also encouraging the development of combination inhalers that can deliver multiple drugs simultaneously, reducing the pill burden and improving adherence. From a design perspective, portability, intuitive user interfaces, and tamper-resistant packaging are becoming standard expectations. Additionally, the global emphasis on health equity is leading to the development of cost-effective MDIs that can serve patients in low-resource settings without compromising quality. As personalized medicine gains traction, there is increasing interest in customizing inhaler drug formulations and delivery mechanisms based on individual patient profiles, including lung function, age, and disease severity. Healthcare providers are also integrating patient education into treatment plans, which supports the use of smart inhalers that offer real-time usage insights. All these factors are encouraging a more patient-centric approach to MDI development, where functionality, comfort, and feedback are key priorities.What Are the Key Growth Drivers for the Metered Dose Inhalers Market?
The growth in the metered dose inhalers market is driven by several factors linked to demographic trends, disease prevalence, technological innovation, and healthcare system evolution. The rising global incidence of respiratory diseases such as asthma, COPD, and bronchitis is the most significant driver, particularly in urban environments where pollution and lifestyle factors contribute to chronic respiratory issues. Aging populations in both developed and emerging markets are also expanding the patient base for inhalation therapies. From a technological standpoint, the introduction of smart inhalers with digital health integration is creating new value propositions for healthcare providers and payers alike. These devices offer improved monitoring and adherence, which are crucial in managing chronic diseases and reducing hospital admissions. Additionally, the increasing accessibility of diagnostic tools like spirometry is leading to earlier diagnosis of respiratory conditions, which in turn drives earlier and more consistent use of MDIs. On the manufacturing side, the entry of generic and low-cost alternatives is expanding availability across different economic segments, especially in Asia, Latin America, and Africa. Government initiatives aimed at universal healthcare access and chronic disease management are also fueling demand. Moreover, as environmental regulations pressure companies to adopt greener propellant technologies, those that successfully innovate in this area are likely to capture greater market share. The ongoing shift toward outpatient care and home-based treatment further reinforces the role of MDIs as essential, self-administered therapeutic tools. Finally, increased investment in R&D and public-private collaborations aimed at combating respiratory health challenges globally are accelerating product development and distribution. Together, these dynamics are creating a robust and expanding market landscape for metered dose inhalers.Report Scope
The report analyzes the Metered Dose Inhalers market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Press Metered Inhalers, Breath Actuated Inhalers); Propellant (HFA 134A Propellant, HFA 227EA Propellant, HFA-152A Propellant); Technology (Pulmonary Platform Technology, Nasal Platform Technology); Indication (Asthma Indication, COPD Indication, Other Indications); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Distribution Channel).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Press Metered Inhalers segment, which is expected to reach US$14.6 Billion by 2030 with a CAGR of a 6.5%. The Breath Actuated Inhalers segment is also set to grow at 3.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.4 Billion in 2024, and China, forecasted to grow at an impressive 8.8% CAGR to reach $4.5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Metered Dose Inhalers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Metered Dose Inhalers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Metered Dose Inhalers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amada Co., Ltd., ANDRITZ Group, DMG MORI, Esab, FANUC Corp. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this Metered Dose Inhalers market report include:
- 3M Pharmaceuticals Pty Ltd
- AptarGroup, Inc.
- Aristo Pharma Ltd
- AstraZeneca plc
- Beximco Pharma Ltd
- Bespak Europe
- Boehringer Ingelheim
- Biocare Manufacturing Sdn Bhd
- Cipla Inc
- GlaxoSmithKline plc (GSK)
- Globe Medical
- H&T Presspart Manufacturing Ltd
- Intech Biopharm Corporation
- Midascare Pharmaceuticals Pvt Ltd
- Mylan N.V.
- Novartis AG
- Opko Health, Inc.
- Presspart Manufacturing Ltd
- Propeller Health
- Sunovion Pharmaceuticals Inc.
- Vectura Group plc
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Pharmaceuticals Pty Ltd
- AptarGroup, Inc.
- Aristo Pharma Ltd
- AstraZeneca plc
- Beximco Pharma Ltd
- Bespak Europe
- Boehringer Ingelheim
- Biocare Manufacturing Sdn Bhd
- Cipla Inc
- GlaxoSmithKline plc (GSK)
- Globe Medical
- H&T Presspart Manufacturing Ltd
- Intech Biopharm Corporation
- Midascare Pharmaceuticals Pvt Ltd
- Mylan N.V.
- Novartis AG
- Opko Health, Inc.
- Presspart Manufacturing Ltd
- Propeller Health
- Sunovion Pharmaceuticals Inc.
- Vectura Group plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 558 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
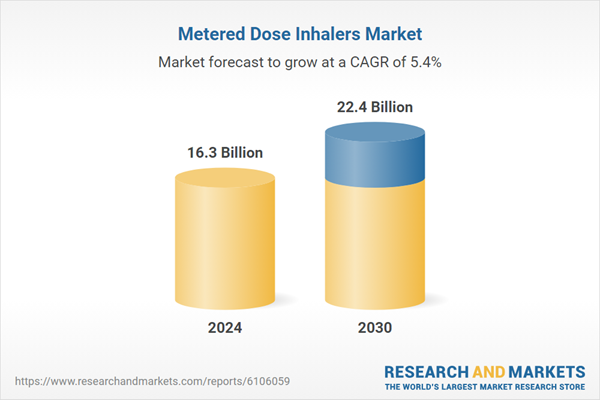
| Estimated Market Value ( USD | $ 16.3 Billion |
| Forecasted Market Value ( USD | $ 22.4 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |