Global Mpox Diagnostics Market - Key Trends & Drivers Summarized
Why Is There Renewed Focus on Diagnostic Solutions for Mpox?
Mpox diagnostics have gained renewed urgency due to changing patterns in disease outbreaks, increasing global surveillance efforts, and a shift in case detection priorities. Earlier, mpox was largely endemic to specific regions, but recent cross-border transmission has made timely diagnosis a public health priority. Diagnosis plays a central role in early case isolation, outbreak tracking, and contact tracing. To ensure speed and accuracy, diagnostic tools now focus on lesion-based sampling rather than traditional serological approaches.Laboratory testing continues to rely primarily on nucleic acid amplification methods. These techniques detect the presence of viral DNA in skin lesions and scabs. Testing accuracy is highest when samples are collected from active lesions, avoiding potential variability in viral load from blood samples. Focus has shifted toward tools that allow rapid, reliable, and decentralized testing, particularly in regions with limited access to advanced laboratory infrastructure. Simplification of sample collection and faster processing times are increasingly important design factors for new diagnostic platforms.
What Makes PCR-Based Testing the Benchmark in Current Practice?
PCR-based testing remains the foundation for accurate mpox diagnosis. This method is highly sensitive and specific, detecting even low quantities of viral DNA. Real-time PCR assays are currently the most trusted platform used in clinical settings and public health laboratories. These tests target specific regions of the mpox virus genome, allowing differentiation between clades and confirmation of infection status. Several types of PCR systems exist, ranging from lab-based analyzers to portable, cartridge-based units designed for point-of-care use.New molecular diagnostic platforms are emerging to improve access and reduce turnaround times. Some allow testing in under one hour, supporting faster decision-making in outbreak settings. These systems are particularly useful where rapid patient triage and isolation are needed. In addition to centralized testing, portable molecular platforms are gaining relevance for use in field hospitals, border entry points, and mobile health units. Emphasis remains on operational simplicity, minimal technical training, and compatibility with existing healthcare workflows.
Why Have Rapid Antigen Tests Failed to Meet Diagnostic Standards?
Antigen-based rapid diagnostic tests have not yet proven reliable for mpox detection. While some show high specificity, their sensitivity is consistently low. Most antigen tests struggle to detect lower viral loads, especially in early or healing stages of infection. Their inability to detect asymptomatic or pre-symptomatic cases limits their use in surveillance or early response. This limitation restricts their role to supplementary screening rather than standalone diagnosis. Due to these constraints, health authorities do not currently endorse these tests for clinical use.In response to these gaps, development efforts are shifting toward improved molecular tools and novel detection technologies. Some diagnostic tools now combine molecular detection with digital readouts or remote monitoring features. Experimental systems using AI-assisted image recognition to assess lesion photographs are also being evaluated. These tools aim to support diagnosis in remote locations where laboratory facilities are unavailable. However, such systems require further clinical validation before they can be used widely.
What Factors Are Driving Growth in the Mpox Diagnostics Market?
Growth in the mpox diagnostics market is driven by several factors. Rising frequency of outbreaks and their geographic spread has increased the demand for faster detection tools. Expanded use of point-of-care PCR devices is helping reduce testing delays in rural and low-resource settings. Integration of molecular diagnostics into outbreak response kits is enabling early containment. Increased investment in portable platforms that operate without extensive laboratory infrastructure is encouraging market participation from new manufacturers.Greater emphasis on decentralized testing and mobile diagnostics is aligning with public health goals in developing countries. Uptake of digital health tools that link test results with surveillance databases is also contributing to efficient outbreak management. Growing need for differential diagnosis due to overlapping symptoms with other skin or febrile illnesses is raising the importance of high-specificity assays. Ongoing product approvals and regulatory guidance for emergency use of new diagnostic platforms continue to support product innovation and regional accessibility.
Report Scope
The report analyzes the Mpox Diagnostics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Diagnostic Test (Antigen Detection Test, Molecular Test); Sample (Urine Sample, Whole Blood Sample, Skin Lesion Specimen Sample).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Antigen Detection Test segment, which is expected to reach US$1.5 Billion by 2030 with a CAGR of a 2.1%. The Molecular Test segment is also set to grow at 4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $515.4 Million in 2024, and China, forecasted to grow at an impressive 5% CAGR to reach $426.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Mpox Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Mpox Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Mpox Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acer, Corsair, Elecom, Glorious PC Gaming Race, HyperX and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Mpox Diagnostics market report include:
- Abbott Laboratories
- ACON Biotech (Hangzhou)
- Altona Diagnostics GmbH
- Bio-Rad Laboratories, Inc.
- bioMérieux
- Cepheid (Danaher Corporation)
- Creative Biogene Inc.
- DiaSorin S.p.A.
- Hologic, Inc.
- Labcorp
- Mayo Clinic Laboratories
- Meridian Bioscience
- OraSure Technologies
- PerkinElmer Inc.
- Pfizer (Alinity™ m MPXV assay)
- Qiagen N.V.
- Quidel Corporation
- Roche Diagnostics
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
- Quest Diagnostics
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- ACON Biotech (Hangzhou)
- Altona Diagnostics GmbH
- Bio-Rad Laboratories, Inc.
- bioMérieux
- Cepheid (Danaher Corporation)
- Creative Biogene Inc.
- DiaSorin S.p.A.
- Hologic, Inc.
- Labcorp
- Mayo Clinic Laboratories
- Meridian Bioscience
- OraSure Technologies
- PerkinElmer Inc.
- Pfizer (Alinity™ m MPXV assay)
- Qiagen N.V.
- Quidel Corporation
- Roche Diagnostics
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
- Quest Diagnostics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 268 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
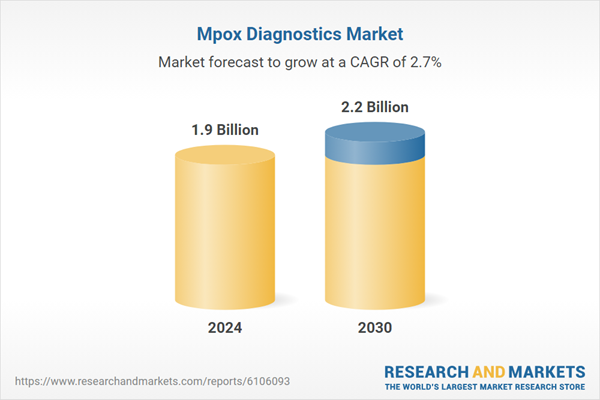
| Estimated Market Value ( USD | $ 1.9 Billion |
| Forecasted Market Value ( USD | $ 2.2 Billion |
| Compound Annual Growth Rate | 2.7% |
| Regions Covered | Global |