Global Hemodynamic Monitoring Devices Market - Key Trends & Drivers Summarized
Is Hemodynamic Monitoring Entering a New Era of Precision Medicine?
Hemodynamic monitoring-the continuous assessment of cardiovascular parameters like blood pressure, cardiac output, and tissue perfusion-is becoming increasingly central to the practice of precision medicine, particularly in critical care, emergency medicine, and perioperative settings. Historically, hemodynamic monitoring relied heavily on invasive catheter-based methods such as the pulmonary artery catheter (PAC), which, despite its diagnostic utility, presented significant risks including infection, thrombosis, and vascular damage. However, recent years have witnessed a significant paradigm shift toward less invasive and noninvasive technologies that provide real-time, accurate, and actionable data with lower complication rates. Technologies such as pulse contour analysis, bioimpedance, bioreactance, and Doppler ultrasound are gaining traction as effective tools for dynamic and patient-specific cardiovascular assessment. These methods allow clinicians to tailor fluid therapy, vasopressor support, and inotropic treatment to each patient's individual hemodynamic profile, thereby reducing complications and improving outcomes. The integration of hemodynamic monitoring into electronic health records and clinical decision-support systems is enabling predictive analytics and trend-based diagnostics, further aligning with the goals of personalized care. Innovations such as wearable hemodynamic monitors and wireless telemetry systems are also pushing the boundaries of care beyond the ICU, allowing for remote monitoring in step-down units, outpatient care, and even home settings. This evolution is driven by the broader healthcare trend toward minimizing invasiveness while maximizing data richness, and it marks a transformative era in which cardiovascular monitoring is both safer and more clinically effective than ever before.Are Noninvasive and Minimally Invasive Technologies Disrupting Traditional Monitoring Methods?
Noninvasive and minimally invasive hemodynamic monitoring devices are rapidly gaining ground in the clinical landscape, challenging the long-standing dominance of catheter-based systems. This disruption is rooted in the growing demand for safer, faster, and more comfortable diagnostic tools that maintain or exceed the accuracy of traditional methods. Pulse wave transit time, thoracic electrical bioimpedance, and volume clamp technologies are now providing continuous blood pressure and cardiac output data without the need for arterial or venous catheterization. These systems are particularly valuable in perioperative care, where rapid assessments of fluid responsiveness and cardiac function can drastically influence surgical outcomes and recovery times. For instance, anesthesiologists are increasingly adopting esophageal Doppler and finger cuff technologies during major surgeries to maintain optimal perfusion without invasive line placements. Additionally, these innovations are playing a critical role in managing fragile patient populations-such as neonates, the elderly, and those with coagulopathies-where the risk of invasive monitoring outweighs its benefits. Many noninvasive systems are now equipped with intuitive interfaces, real-time data visualization, and wireless connectivity, making them accessible to a broader range of healthcare providers, including emergency medical technicians, nurses, and remote monitoring personnel. Furthermore, clinical trials continue to validate the effectiveness of these tools, encouraging guideline updates and broader adoption across hospitals and ambulatory care settings. Startups and major medical device companies alike are investing heavily in noninvasive technologies, spurred by the global shift toward value-based healthcare and patient-centered service models. As these technologies continue to mature, they promise to redefine standard monitoring protocols while expanding access to critical cardiovascular diagnostics in both advanced and resource-limited settings.Is the Demand for Advanced Critical Care and Perioperative Monitoring Driving Market Expansion?
The rising complexity and acuity of patients in critical care units and operating rooms are creating strong demand for advanced hemodynamic monitoring solutions capable of delivering real-time, actionable insights. In intensive care units (ICUs), where time-sensitive interventions can be the difference between life and death, accurate assessment of cardiac output, preload, afterload, and oxygen delivery is indispensable. These parameters inform crucial decisions on fluid resuscitation, vasoactive medication titration, and mechanical circulatory support, especially in conditions such as sepsis, cardiogenic shock, and multi-organ failure. Hemodynamic monitoring is also becoming increasingly central to enhanced recovery after surgery (ERAS) protocols, where goal-directed fluid therapy (GDFT) has shown to reduce complications, shorten hospital stays, and lower costs. In high-risk surgeries such as cardiac, thoracic, and major abdominal procedures, intraoperative hemodynamic optimization is directly correlated with improved postoperative outcomes, driving the uptake of advanced monitoring devices. In addition, the growing volume of surgeries among aging populations and patients with comorbidities is prompting hospitals to invest in more sophisticated intraoperative and postoperative monitoring systems. Technologies that allow continuous beat-to-beat analysis of cardiac output, systemic vascular resistance, and stroke volume variation are now preferred over intermittent measurements, providing clinicians with deeper insights into patient physiology. The global growth of tertiary care hospitals and surgical centers-particularly in Asia-Pacific, Latin America, and the Middle East-is also fueling demand for scalable and integrated monitoring systems. Moreover, hospital administrators are increasingly prioritizing technologies that support resource efficiency and reduce readmissions, further supporting the market for hemodynamic monitoring devices that enhance early detection and proactive intervention.What Are the Key Drivers Accelerating Growth in the Hemodynamic Monitoring Devices Market?
The growth in the hemodynamic monitoring devices market is driven by several factors related to technological innovation, evolving clinical practices, healthcare infrastructure expansion, and changing patient demographics. One of the most prominent drivers is the increasing global prevalence of cardiovascular diseases, sepsis, and critically ill patients requiring continuous monitoring, especially in aging populations. In tandem, the demand for perioperative monitoring is surging due to a rise in elective and high-risk surgeries worldwide. Technological advancements in sensor miniaturization, real-time data analytics, wireless communication, and user interface design have made hemodynamic devices more accurate, user-friendly, and deployable across various clinical environments-from ICUs and operating rooms to emergency departments and remote care facilities. Additionally, the healthcare industry`s transition toward value-based care is emphasizing early diagnosis, real-time treatment adjustments, and patient safety-all of which benefit from high-performance monitoring systems. Clinical guidelines are increasingly endorsing the use of advanced monitoring for goal-directed therapy in critical care and surgery, encouraging hospitals and clinicians to adopt new technologies. Reimbursement policies are also evolving to support these technologies, especially in developed markets where outcome-based payments are becoming the norm. Meanwhile, emerging economies are ramping up investment in healthcare infrastructure, and as they build new hospitals and expand ICU capabilities, demand for hemodynamic devices is rising in parallel. Another important driver is the digital transformation of healthcare, which is integrating monitoring data into electronic health records and cloud platforms to support remote diagnostics, tele-ICU models, and machine learning-based predictive analytics. Lastly, growing awareness among clinicians and healthcare administrators about the role of precise hemodynamic data in improving clinical outcomes is cementing the value of these devices across the care continuum. These combined forces are driving sustained growth and innovation in a market poised to become a cornerstone of modern critical care and perioperative medicine.Scope Of Study:
The report analyzes the Hemodynamic Monitoring Devices market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Product (Hemodynamic Monitoring Disposables, Hemodynamic Monitors); System Type (Invasive System, Minimally Invasive System, Non-Invasive System); End-Use (Hospitals End-Use, Catheterization Labs End-Use, Other End-Uses)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Hemodynamic Monitoring Disposables segment, which is expected to reach US$1.7 Billion by 2030 with a CAGR of a 6.8%. The Hemodynamic Monitors segment is also set to grow at 3.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $475.8 Million in 2024, and China, forecasted to grow at an impressive 9.4% CAGR to reach $501.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hemodynamic Monitoring Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hemodynamic Monitoring Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hemodynamic Monitoring Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, B. Braun Melsungen AG, Baxter International Inc., Bistos Co. Ltd., Caretaker Medical and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Hemodynamic Monitoring Devices market report include:
- Abbott Laboratories
- B. Braun Melsungen AG
- Baxter International Inc.
- Bistos Co. Ltd.
- Caretaker Medical
- CNSystems Medizintechnik GmbH
- Deltex Medical Group plc
- Drägerwerk AG & Co. KGaA
- Edwards Lifesciences Corporation
- GE Healthcare
- Getinge AB
- ICU Medical, Inc.
- LiDCO Group plc
- Masimo Corporation
- Medtronic plc
- Mindray Medical International Ltd.
- Nihon Kohden Corporation
- Osypka Medical GmbH
- Philips Healthcare
- PULSION Medical Systems SE
- Siemens Healthineers AG
- Uscom Ltd.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- B. Braun Melsungen AG
- Baxter International Inc.
- Bistos Co. Ltd.
- Caretaker Medical
- CNSystems Medizintechnik GmbH
- Deltex Medical Group plc
- Drägerwerk AG & Co. KGaA
- Edwards Lifesciences Corporation
- GE Healthcare
- Getinge AB
- ICU Medical, Inc.
- LiDCO Group plc
- Masimo Corporation
- Medtronic plc
- Mindray Medical International Ltd.
- Nihon Kohden Corporation
- Osypka Medical GmbH
- Philips Healthcare
- PULSION Medical Systems SE
- Siemens Healthineers AG
- Uscom Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 372 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
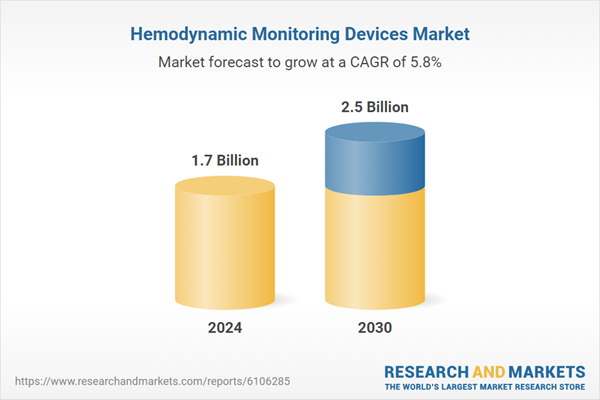
| Estimated Market Value in 2024 | 1.7 Billion |
| Forecasted Market Value by 2030 | 2.5 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |