Global Human Immunoglobulin Market - Key Trends & Drivers Summarized
How Is the Expanding Scope of Immune-Related Disorders Shaping the Use of Human Immunoglobulin?
Human immunoglobulin, a vital blood-derived product rich in antibodies, is increasingly being deployed across a wide range of therapeutic areas, particularly in the management of immune deficiencies and autoimmune disorders. As the global burden of immune-related diseases grows, medical professionals are turning to immunoglobulin therapy for both replacement and immunomodulatory purposes. Primary immunodeficiency disorders, where patients lack the natural ability to produce sufficient antibodies, are among the most common indications for these therapies. In such cases, immunoglobulin infusions are crucial for preventing life-threatening infections and ensuring long-term health stability. The product is also used extensively to treat autoimmune neurological diseases such as chronic inflammatory demyelinating polyneuropathy and myasthenia gravis, where it helps to regulate an overactive immune response. Furthermore, secondary immunodeficiencies caused by chemotherapy, HIV, or bone marrow transplantation are also being managed with human immunoglobulin therapy, highlighting its versatility. The growing awareness among healthcare providers and patients regarding these applications has resulted in a steady increase in demand across hospitals, specialty clinics, and home care settings. As disease awareness rises and diagnostic capabilities improve, more individuals are being correctly identified as candidates for this treatment, further reinforcing the clinical reliance on human immunoglobulin. This expanding therapeutic scope is not only elevating the medical significance of immunoglobulin but is also laying the groundwork for sustained market growth.Why Are Technological Advancements in Plasma Collection and Purification Enhancing Supply and Safety?
Technological innovation in plasma collection and immunoglobulin purification is playing a transformative role in shaping the reliability, safety, and availability of these vital therapies. The production of human immunoglobulin begins with the collection of plasma from healthy donors, and the introduction of automated apheresis machines has improved both the efficiency and yield of this process. These machines allow for selective extraction of plasma components, reducing donor fatigue while increasing output. Once collected, the plasma undergoes rigorous purification involving processes such as ethanol fractionation, chromatography, and viral inactivation to ensure the removal of pathogens and impurities. These steps are continually being refined to enhance safety without compromising the efficacy of the final product. New filtration methods are allowing manufacturers to achieve higher levels of purity, reducing the risk of adverse reactions in recipients. Moreover, innovations in cold-chain logistics and packaging are improving the stability and shelf life of immunoglobulin preparations, especially important in regions with limited access to refrigerated transport. Many producers are also investing in recombinant technologies and alternative expression systems, though human plasma remains the primary source. These advancements collectively ensure that the supply chain for immunoglobulin is becoming more robust, scalable, and responsive to global demand fluctuations. As more countries seek to establish or expand their plasma collection networks, these technologies provide the blueprint for safer and more efficient immunoglobulin therapies worldwide.How Are Healthcare Infrastructure and Policy Initiatives Supporting Broader Access to Immunoglobulin Therapy?
Access to human immunoglobulin therapy is being increasingly supported by national healthcare initiatives, international collaborations, and evolving reimbursement structures. In high-income countries, immunoglobulin is generally included in public or private insurance plans, allowing widespread clinical use in hospitals and outpatient care facilities. In recent years, governments in middle-income countries have also begun integrating immunoglobulin into essential medicines lists, recognizing its value in treating immune-related conditions that often go underdiagnosed. Some nations are implementing plasma self-sufficiency programs to reduce reliance on imported products and improve availability through domestic production. Public-private partnerships and nonprofit organizations are facilitating infrastructure development, especially in regions where plasma collection and processing capacity have been historically limited. Additionally, disease-specific advocacy groups have played a crucial role in pushing for equitable access and policy reforms that prioritize timely diagnosis and long-term treatment. Standardized treatment guidelines and updated protocols from international health bodies are also helping physicians around the world understand when and how to use immunoglobulin effectively. Improvements in training and education for healthcare professionals, combined with greater access to diagnostic tools, are ensuring that patients receive appropriate therapy based on evidence-based indications. These systemic efforts are breaking down traditional barriers to immunoglobulin therapy, expanding its reach beyond tertiary hospitals to smaller clinics and rural health centers. As healthcare systems continue to mature and become more inclusive, the infrastructure supporting immunoglobulin use is becoming more reliable, equitable, and aligned with global standards of care.What Key Factors Are Driving the Global Expansion of the Human Immunoglobulin Market?
The global expansion of the human immunoglobulin market is driven by a confluence of medical, technological, demographic, and strategic factors that reflect growing demand and evolving healthcare priorities. One of the primary drivers is the increasing prevalence of immune deficiencies, both congenital and acquired, coupled with rising diagnostic rates due to improved medical awareness and screening capabilities. An aging global population is also contributing to market growth, as elderly individuals are more susceptible to infections and autoimmune conditions, making them key beneficiaries of immunoglobulin therapy. The rapid advancement of plasma collection and fractionation technology ensures a more stable supply chain, which is essential given the high resource intensity of immunoglobulin production. In parallel, regulatory frameworks are becoming more supportive, with expedited approvals and standardized safety requirements making it easier for new products to enter the market. Pharmaceutical companies are also focusing on the development of subcutaneous formulations and other patient-friendly delivery systems that support home-based care and improve treatment adherence. Strategic investments and mergers in the biopharmaceutical sector are enabling broader distribution networks and deeper market penetration, especially in emerging economies. Additionally, the growing focus on rare diseases and orphan drug incentives is encouraging innovation in niche immunoglobulin therapies for less common indications. As awareness campaigns, clinical training, and government support continue to expand, the human immunoglobulin market is positioned for sustained global growth, offering critical solutions for patients whose immune systems require reliable, effective support.Report Scope
The report analyzes the Human Immunoglobulin market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Autoimmune Disorders Application, Hematology Diseases Application, Inflammatory Diseases Application, Infectious Diseases Application, Other Applications); End-Use (Hospitals End-Use, Clinics End-Use, Ambulatory Surgery Centers End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Autoimmune Disorders Application segment, which is expected to reach US$12.6 Billion by 2030 with a CAGR of a 10.7%. The Hematology Diseases Application segment is also set to grow at 6.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.6 Billion in 2024, and China, forecasted to grow at an impressive 14.1% CAGR to reach $6.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Human Immunoglobulin Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Human Immunoglobulin Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Human Immunoglobulin Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Bayer AG, Bharat Serums and Vaccines Ltd, Biomed Pvt. Ltd., Bristol Myers Squibb and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Human Immunoglobulin market report include:
- ADMA Biologics, Inc.
- Baxter International Inc.
- Bharat Serums and Vaccines Ltd
- Biotest AG
- Bio Products Laboratory Ltd (BPL)
- CSL Behring
- China National Pharmaceutical Group (Sinopharm)
- GC Pharma
- Grifols, S.A.
- Hualan Biological Engineering Inc.
- Intas Pharmaceuticals Ltd.
- Kedrion Biopharma
- LFB S.A.
- Octapharma AG
- Reliance Life Sciences
- Sanquin
- Shanghai RAAS Blood Products Co.
- Shire (Now part of Takeda)
- Sichuan Yuanda Shuyang Pharmaceutical Co.
- Takeda Pharmaceutical Company Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ADMA Biologics, Inc.
- Baxter International Inc.
- Bharat Serums and Vaccines Ltd
- Biotest AG
- Bio Products Laboratory Ltd (BPL)
- CSL Behring
- China National Pharmaceutical Group (Sinopharm)
- GC Pharma
- Grifols, S.A.
- Hualan Biological Engineering Inc.
- Intas Pharmaceuticals Ltd.
- Kedrion Biopharma
- LFB S.A.
- Octapharma AG
- Reliance Life Sciences
- Sanquin
- Shanghai RAAS Blood Products Co.
- Shire (Now part of Takeda)
- Sichuan Yuanda Shuyang Pharmaceutical Co.
- Takeda Pharmaceutical Company Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 279 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
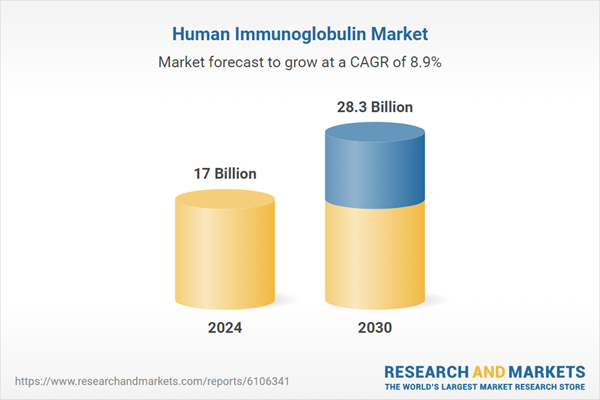
| Estimated Market Value ( USD | $ 17 Billion |
| Forecasted Market Value ( USD | $ 28.3 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |