Global Needle-Free Diabetes Care Market - Key Trends & Drivers Summarized
Why Is Needle-Free Diabetes Care Attracting Attention in Chronic Disease Management?
Needle-free diabetes care technologies are being developed and adopted to address the discomfort, inconvenience, and risks associated with traditional needle-based methods of glucose monitoring and insulin delivery. For many patients, repeated finger-pricking and insulin injections cause pain, skin irritation, and anxiety, which can impact adherence to treatment. Needle-free systems aim to improve patient compliance by offering painless, user-friendly, and non-invasive or minimally invasive alternatives.These technologies include transdermal patches, jet injectors, laser-based devices, microneedle arrays, and non-invasive glucose monitors that use interstitial fluid, sweat, or optical signals. Wearable continuous glucose monitors (CGMs) and smart sensors are increasingly replacing manual monitoring. Devices that integrate insulin delivery with monitoring capabilities are also emerging, allowing for more precise and personalized glycemic control. The increasing emphasis on quality of life and long-term disease self-management is fueling interest in these innovative tools.
How Are Emerging Technologies Enhancing Accessibility and Efficiency?
Advancements in biosensor technology, miniaturization, and wireless communication are driving development of more accurate and responsive needle-free solutions. CGMs using skin sensors now transmit real-time data to smartphones or insulin pumps, enabling continuous tracking without physical disruption. Jet injectors, which deliver insulin through high-pressure streams without needles, are being refined to offer better dosing accuracy and reduced tissue trauma.Non-invasive blood glucose monitoring devices using optical, electromagnetic, or ultrasound signals are under active research, although achieving reliable accuracy remains a challenge. Digital health platforms are increasingly linked with these devices, allowing remote monitoring by healthcare providers and integration into broader chronic care ecosystems. Battery efficiency, user interface design, and data visualization tools are being improved to support daily usability and broader adoption.
Which End-Users and Care Models Are Driving Demand for Needle-Free Solutions?
The primary users of needle-free diabetes devices include type 1 and type 2 diabetes patients seeking comfort, convenience, and better treatment adherence. Pediatric patients, elderly individuals, and needle-phobic users are particularly likely to benefit from non-invasive options. Home-based and remote care models are helping expand demand for self-operated and wearable solutions that minimize clinic visits while maintaining control.Healthcare providers and diabetes educators are also incorporating needle-free devices into patient management plans to improve engagement and reduce complications. Pharmacies, telemedicine platforms, and insurance providers are beginning to support these devices as part of personalized health plans. In regions with high diabetes prevalence and limited clinical infrastructure, needle-free technologies offer scalable alternatives that reduce the burden on traditional healthcare systems.
Growth in the Needle-Free Diabetes Care Market Is Driven by Several Factors…
Growth in the needle-free diabetes care market is driven by several factors. Increasing diabetes prevalence worldwide demands better and more patient-friendly management tools. Advancements in sensor technology, wearable electronics, and wireless data integration improve device performance and user experience. Rising awareness of self-monitoring and long-term disease control supports consumer adoption. Expansion of digital health ecosystems encourages the integration of needle-free devices with mobile apps and cloud platforms. Pediatric and geriatric care models favor painless solutions that improve safety and compliance. Regulatory approvals and investment in non-invasive diagnostics are accelerating product development and market entry across multiple regions.Report Scope
The report analyzes the Needle Free Diabetes Care market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Device Type (Insulin Infuser, Insulin Patches, Insulin Pens, Insulin Pumps, Jet Injectors); End-Use (Homecare End-Use, Diagnostic Centers End-Use, Hospitals & Clinics End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Insulin Infuser segment, which is expected to reach US$5.6 Billion by 2030 with a CAGR of a 3.2%. The Insulin Patches segment is also set to grow at 5.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.6 Billion in 2024, and China, forecasted to grow at an impressive 7.5% CAGR to reach $3.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Needle Free Diabetes Care Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Needle Free Diabetes Care Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Needle Free Diabetes Care Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Beurer GmbH, BODYFRIEND, Breo, Casada, Cozzia and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Needle Free Diabetes Care market report include:
- 3M Company
- Akeso/D. Medical Industries (Spring pump)
- Akra Dermojet
- Antares Pharma, Inc.
- Bioject Medical Technologies, Inc.
- CeQur Corporation
- Crossject SA
- Echo Therapeutics Inc.
- Endo International plc
- European Pharma Group BV
- Injex Pharma AG
- Insulet Corporation (Omnipod)
- Johnson & Johnson
- MannKind Corporation
- Medtronic PLC
- NuGen Medical Devices (InsuJet)
- Pancreum LLC
- PharmaJet, Inc.
- Tandem Diabetes Care, Inc.
- Valeritas, Inc.
- Zogenix, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Company
- Akeso/D. Medical Industries (Spring pump)
- Akra Dermojet
- Antares Pharma, Inc.
- Bioject Medical Technologies, Inc.
- CeQur Corporation
- Crossject SA
- Echo Therapeutics Inc.
- Endo International plc
- European Pharma Group BV
- Injex Pharma AG
- Insulet Corporation (Omnipod)
- Johnson & Johnson
- MannKind Corporation
- Medtronic PLC
- NuGen Medical Devices (InsuJet)
- Pancreum LLC
- PharmaJet, Inc.
- Tandem Diabetes Care, Inc.
- Valeritas, Inc.
- Zogenix, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 284 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
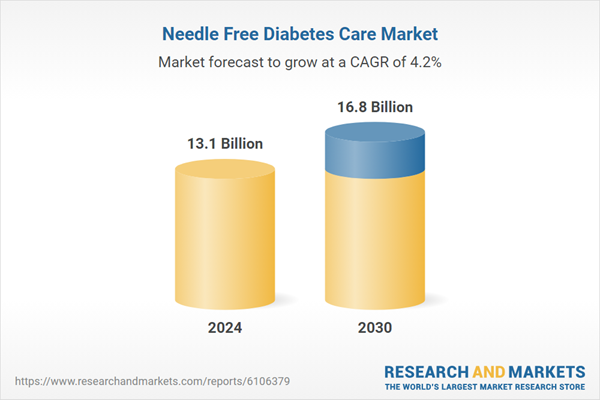
| Estimated Market Value ( USD | $ 13.1 Billion |
| Forecasted Market Value ( USD | $ 16.8 Billion |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | Global |