Global Norovirus Diagnostics Market - Key Trends & Drivers Summarized
Why Is Demand for Norovirus Testing Increasing in Healthcare Settings?
Norovirus is one of the most common causes of viral gastroenteritis worldwide, affecting individuals across all age groups. Due to its high transmissibility, outbreaks often occur in hospitals, long-term care facilities, schools, and cruise ships. The need for timely and accurate diagnosis has increased as healthcare systems aim to reduce the spread of infection and manage outbreaks efficiently. Unlike other gastrointestinal illnesses, norovirus can spread quickly through contaminated surfaces, food, or water, making early identification critical.Routine diagnostic practices now incorporate norovirus testing, especially for patients presenting with acute diarrhea in institutional settings. Clinicians increasingly require rapid, sensitive tools to differentiate norovirus from bacterial and other viral infections. The growing adoption of diagnostic testing in outpatient care and emergency settings reflects heightened clinical awareness and regulatory emphasis on infection control protocols.
How Are Diagnostic Technologies Evolving to Improve Speed and Accuracy?
Technological advancements are driving the shift from traditional enzyme immunoassays toward molecular diagnostics that offer greater sensitivity and specificity. Real-time PCR-based assays and multiplex panels are now widely used for detecting norovirus genogroups in stool samples. These platforms deliver rapid results, enabling healthcare providers to take timely preventive measures and initiate appropriate care.Point-of-care testing devices are also emerging for decentralized use in clinics and care facilities, providing faster turnaround without the need for centralized labs. Improvements in assay design, sample preparation, and nucleic acid amplification are supporting reliable detection of low viral loads. Multiplex molecular tests that include norovirus along with other enteric pathogens are increasingly preferred in clinical workflows to streamline diagnosis in gastroenteritis cases.
What Institutional and Public Health Trends Are Expanding Testing Adoption?
Institutions such as hospitals, schools, and elder care centers are investing in routine norovirus screening protocols to reduce the impact of outbreaks. Public health agencies have implemented stricter guidelines for managing acute gastroenteritis in high-risk environments, contributing to more frequent diagnostic testing. Laboratories are adopting automated systems that allow high-throughput norovirus detection during peak seasons or in outbreak scenarios.Global travel and food service industries also play a role, as foodborne and waterborne transmission drive demand for diagnostic surveillance. In response, food safety laboratories and environmental testing services are incorporating norovirus assays to meet compliance and quality control standards. Surveillance programs tracking genotypic variation and outbreak sources rely heavily on laboratory-confirmed norovirus cases, further integrating diagnostics into broader epidemiological monitoring systems.
Growth in the norovirus diagnostics market is driven by several factors.
Rising incidence of norovirus-related outbreaks in institutional and community settings is pushing demand for rapid and accurate diagnostic tools. Technological improvements in molecular testing, including real-time PCR and multiplex platforms, are enhancing detection speed and reliability. Increasing implementation of infection control protocols in hospitals, long-term care facilities, and schools is encouraging more consistent use of diagnostics. Expansion of point-of-care testing and high-throughput lab platforms is supporting faster outbreak response. Regulatory focus on food safety and public health surveillance is further driving adoption of norovirus diagnostics across healthcare, environmental, and food industry applications.Report Scope
The report analyzes the Norovirus Diagnostics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Rapid Test Kits, PCR Kits, ELISA-based Kits); End-Use (Hospitals End-Use, Diagnostics Labs End-Use, Clinics End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Rapid Test Kits segment, which is expected to reach US$68.3 Million by 2030 with a CAGR of a 7.9%. The PCR Kits segment is also set to grow at 5.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $18.1 Million in 2024, and China, forecasted to grow at an impressive 7.2% CAGR to reach $17 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Norovirus Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Norovirus Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Norovirus Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ahlstrom Oyj, Alkegen (Lydall/Unifrax), Avgol, Berry Global Inc., DuPont and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Norovirus Diagnostics market report include:
- Abbott Laboratories
- Abingdon Health
- Altona Diagnostics GmbH
- bioMérieux SA
- Becton, Dickinson and Company
- CerTest Biotec S.L.
- Danaher Corporation (Cepheid)
- DiaSorin S.p.A.
- ELITechGroup AG
- Eiken Chemical Co.
- Elisabeth Pharmacon
- Meridian Bioscience, Inc.
- Qiagen N.V.
- Quidel Corporation
- Roche Diagnostics (F. Hoffmann-La Roche Ltd.)
- Seegene Inc.
- Thermo Fisher Scientific, Inc.
- Luminex Corporation
- Bio-Rad Laboratories, Inc.
- Hologic, Inc.
- GenMark Diagnostics, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Abingdon Health
- Altona Diagnostics GmbH
- bioMérieux SA
- Becton, Dickinson and Company
- CerTest Biotec S.L.
- Danaher Corporation (Cepheid)
- DiaSorin S.p.A.
- ELITechGroup AG
- Eiken Chemical Co.
- Elisabeth Pharmacon
- Meridian Bioscience, Inc.
- Qiagen N.V.
- Quidel Corporation
- Roche Diagnostics (F. Hoffmann-La Roche Ltd.)
- Seegene Inc.
- Thermo Fisher Scientific, Inc.
- Luminex Corporation
- Bio-Rad Laboratories, Inc.
- Hologic, Inc.
- GenMark Diagnostics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 132 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
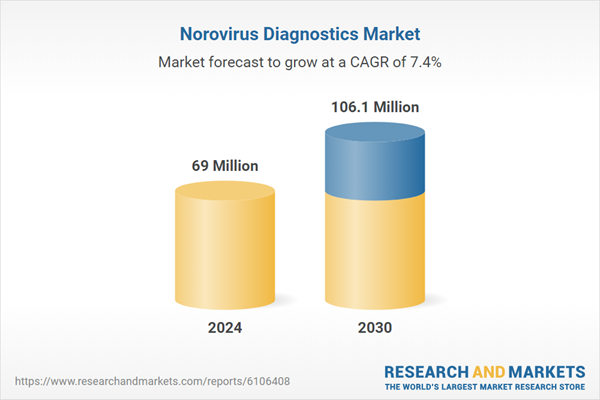
| Estimated Market Value ( USD | $ 69 Million |
| Forecasted Market Value ( USD | $ 106.1 Million |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |