Global Phage Therapy Market - Key Trends & Drivers Summarized
Can Bacteriophages Offer a New Frontier in the Fight Against Antibiotic Resistance?
The escalating global crisis of antibiotic resistance has placed phage therapy under the spotlight as a promising alternative for combating multidrug-resistant bacterial infections. Bacteriophages, or phages, are viruses that selectively infect and destroy bacteria, offering a highly targeted approach without disturbing beneficial microbiota. Unlike traditional antibiotics, which operate on broad-spectrum mechanisms, phages can be tailored to attack specific bacterial strains, thereby minimizing collateral damage and reducing the risk of resistance development. As antibiotic-resistant superbugs continue to threaten public health globally, the appeal of phage therapy is growing not only in clinical medicine but also in sectors like agriculture, aquaculture, and food safety. Multiple countries are now reevaluating regulatory pathways to facilitate the research and clinical integration of phage-based treatments, especially in cases where conventional antibiotics fail. Notably, compassionate use cases and individualized phage therapy have shown life-saving results in critically ill patients, further boosting interest in this technology. Academic institutions, biotech startups, and public health agencies are increasingly collaborating on global phage banks and sequencing libraries to enhance the availability and customization of phage cocktails. The specificity of phage-host interactions, while challenging, also offers a significant advantage in minimizing unintended effects on the broader ecosystem. With infectious disease control facing new hurdles in a post-antibiotic era, phage therapy is being reevaluated not as an experimental outlier but as a strategic pillar in future antimicrobial protocols.How Is Innovation in Biotechnology Advancing the Clinical Utility of Phage Therapy?
Advancements in genetic engineering, synthetic biology, and bioinformatics are playing a pivotal role in expanding the clinical relevance of phage therapy. Today's phage research is far more sophisticated than in earlier decades, with scientists now able to modify phages to overcome bacterial defenses, enhance lytic efficiency, and avoid immune system detection. CRISPR-Cas systems are being employed to design programmable phages capable of targeting specific resistance genes, making the therapy adaptable to a wide array of infections. Additionally, innovations in sequencing technologies and metagenomics are accelerating the identification of effective phage-bacteria pairs, significantly reducing the time required for personalized treatment planning. Encapsulation techniques and nanoformulations are also emerging to improve phage stability, bioavailability, and targeted delivery to infected tissues. The development of phage cocktails, which combine multiple phage strains, is enhancing spectrum coverage and mitigating resistance development during treatment. Biotechnology companies are working on scalable production and purification methods that meet GMP standards, addressing one of the major hurdles in bringing phage therapies to market. Clinical trials in Europe, North America, and Asia are increasingly investigating the role of phage therapy in treating infections ranging from chronic wounds to cystic fibrosis-associated pathogens. Such technological momentum is transforming phage therapy from a last-resort option into a versatile and scientifically validated modality. With the continued convergence of high-throughput lab tools and AI-driven screening systems, phage-based therapeutics are gaining credibility as a next-generation weapon in precision medicine.What Regulatory and Infrastructure Challenges Must Be Addressed for Widespread Adoption?
Despite its potential, the widespread adoption of phage therapy faces numerous regulatory and infrastructural barriers that need urgent attention. Traditional drug approval frameworks are not well-suited to therapies that require high personalization, such as phage preparations that must be matched to a patient's specific bacterial infection. This lack of regulatory clarity slows clinical development and discourages large-scale investment. Regulatory agencies like the FDA and EMA are gradually exploring adaptive licensing models, including compassionate use and hospital exemptions, but uniform global standards remain elusive. Manufacturing consistency, quality control, and phage characterization present additional challenges, as these biologics must be free of contaminants, genetically stable, and functionally reproducible. Another hurdle lies in the current lack of commercial-scale phage production facilities equipped to meet medical-grade standards. Many institutions still rely on in-house labs or academic setups, which limits output and quality standardization. Additionally, the scarcity of clinical professionals trained in phage therapy impedes its routine adoption in hospitals. Public perception and awareness also lag behind scientific advances, with many still viewing phage therapy as experimental or unconventional. Intellectual property constraints further complicate commercialization, as naturally occurring phages are difficult to patent, reducing the incentive for private sector investment. Cross-border collaborations and global consortia are beginning to address these gaps, but the need for dedicated policies, infrastructure upgrades, and industry-wide education remains critical. Overcoming these regulatory and logistical hurdles is essential to fully unlock the global potential of phage therapy as a mainstream medical tool.What Forces Are Fueling Market Growth and Expanding Therapeutic Applications?
The growth in the phage therapy market is driven by several factors related to technological progress, unmet clinical needs, and evolving patient care models. One of the most significant drivers is the surge in multidrug-resistant infections, which are rendering conventional antibiotics increasingly ineffective and heightening demand for viable alternatives. Simultaneously, the advancement of genomic and proteomic tools has dramatically improved phage identification, customization, and deployment, thereby reducing developmental timelines and boosting therapeutic efficacy. The increasing incidence of chronic and hospital-acquired infections is also fueling demand for targeted antimicrobials that can circumvent traditional resistance mechanisms. On the end-user side, hospitals and specialized infection clinics are beginning to incorporate phage therapy for difficult-to-treat cases, supported by growing evidence from pilot programs and case studies. Biopharmaceutical companies and academic research centers are investing heavily in the creation of phage libraries, predictive modeling software, and scalable manufacturing systems to broaden therapeutic availability. Government and non-profit funding initiatives focused on combating antibiotic resistance are providing critical financial support for R&D in this space. Consumer behavior is also playing a role, with patients increasingly advocating for alternative therapies and personalized medicine approaches that go beyond traditional pharmaceuticals. Additionally, veterinary medicine, agriculture, and aquaculture are emerging as strong application areas where phage-based solutions are being used to reduce antibiotic dependency and improve microbial control. All these factors, combined with an intensifying global focus on infectious disease resilience and healthcare innovation, are positioning phage therapy as a high-potential, multidisciplinary growth sector for the foreseeable future.Scope Of Study:
The report analyzes the Phage Therapy market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Product (Escherichia Coli, Staphylococcus Aureus, Salmonella, Other Products); Application (Animal Health Application, Food Application, Other Applications)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Escherichia Coli segment, which is expected to reach US$69.1 Million by 2030 with a CAGR of a 20.4%. The Staphylococcus Aureus segment is also set to grow at 15.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $11.9 Million in 2024, and China, forecasted to grow at an impressive 17.3% CAGR to reach $19.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Phage Therapy Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Phage Therapy Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Phage Therapy Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Adaptive Phage Therapeutics, Armata Pharmaceuticals, BiomX Ltd., C3J Therapeutics, CYTOBIOTICS Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Phage Therapy market report include:
- Adaptive Phage Therapeutics
- Armata Pharmaceuticals
- BiomX Ltd.
- C3J Therapeutics
- CYTOBIOTICS Inc.
- Eligo Bioscience
- EnBiotix, Inc.
- InnoPhage Ltd.
- Intralytix, Inc.
- Locus Biosciences
- Micreos Human Health
- Nextbiotics
- OmniPhage
- Phagelux Inc.
- Pherecydes Pharma
- Phico Therapeutics Ltd.
- TechnoPhage SA
- TAILF Therapeutics
- The Eliava Foundation
- VyPhI, Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Adaptive Phage Therapeutics
- Armata Pharmaceuticals
- BiomX Ltd.
- C3J Therapeutics
- CYTOBIOTICS Inc.
- Eligo Bioscience
- EnBiotix, Inc.
- InnoPhage Ltd.
- Intralytix, Inc.
- Locus Biosciences
- Micreos Human Health
- Nextbiotics
- OmniPhage
- Phagelux Inc.
- Pherecydes Pharma
- Phico Therapeutics Ltd.
- TechnoPhage SA
- TAILF Therapeutics
- The Eliava Foundation
- VyPhI, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 149 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
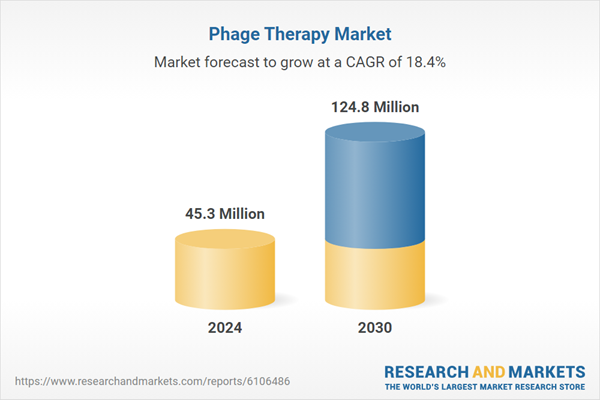
| Estimated Market Value in 2024 | 45.3 Million |
| Forecasted Market Value by 2030 | 124.8 Million |
| Compound Annual Growth Rate | 18.4% |
| Regions Covered | Global |