Global Platelet Rich Plasma Tubes Market - Key Trends & Drivers Summarized
Unveiling the Role of Closed-Tube Centrifugation Systems in Regenerative Medicine and Aesthetic InterventionsWhat Makes Platelet Rich Plasma Tubes Essential to Personalized Cell-Based Therapies?
Platelet rich plasma (PRP) tubes are specialized blood collection containers designed to separate and concentrate autologous platelets from whole blood through centrifugation. These tubes, typically pre-filled with anticoagulants such as sodium citrate and sometimes with thixotropic separation gels, are crucial in obtaining a standardized PRP formulation rich in growth factors, cytokines, and bioactive proteins. PRP tubes are a fundamental component in clinical workflows across orthopedics, dermatology, trichology, dentistry, and aesthetic medicine-where controlled, sterile preparation of platelet concentrates is central to therapeutic efficacy. Two primary categories define the PRP tube landscape: gel-based and gel-free systems. Gel-based tubes incorporate a density-based separator that facilitates clean plasma isolation after centrifugation, while gel-free tubes rely on manual layer extraction post-spin. Tube materials typically include PET plastic or glass, with plastic preferred in high-volume outpatient environments for its shatter-resistance and lighter weight. Most commercially available PRP kits conform to Class II or III medical device classification depending on national regulations, and are often validated for both single-spin and double-spin protocols based on platelet yield requirements. Innovations in cap closure design, color coding, and barcode integration are further streamlining clinical use.Which Applications Are Driving the Demand for PRP Tubes Across Medical Disciplines?
Orthopedics and sports medicine account for a significant share of PRP tube utilization, with growing evidence supporting PRP's role in enhancing tendon healing, osteoarthritis symptom relief, and post-surgical tissue regeneration. PRP injections are commonly administered for rotator cuff tears, tennis elbow, knee osteoarthritis, and plantar fasciitis. In these cases, PRP tubes enable consistent, aseptic concentration of platelets at the point of care, reducing dependency on outsourced laboratories. Clinics offering same-day regenerative interventions have become major procurement hubs for sterile, CE-marked PRP kits. Dermatology and aesthetic practices are rapidly integrating PRP for skin rejuvenation, wrinkle reduction, and acne scar treatment. In this context, PRP tubes support procedures like vampire facials (microneedling + PRP), where patient-derived platelets are topically or intradermally administered to stimulate collagen synthesis. Trichology clinics use PRP for hair restoration in androgenic alopecia, where double-spin PRP protocols yield high platelet concentrations, thereby improving outcomes. Dental surgeons and periodontists also deploy PRP tubes to support bone grafting, implant osseointegration, and soft tissue healing, particularly in complex oral surgery cases.Veterinary applications, particularly in equine medicine, have emerged as a niche growth segment. PRP therapy is being used for ligament injuries, tendon repair, and joint recovery in high-value racehorses, with PRP tubes adapted for larger blood volumes and centrifugation parameters. Meanwhile, academic research institutions exploring PRP's antimicrobial and angiogenic properties are creating baseline demand for precision-engineered PRP tubes in experimental protocols. Across all these segments, the shift toward in-office regenerative procedures has made ease-of-use, sterile certification, and reproducibility vital tube selection criteria.
How Are PRP Tube Designs and Technologies Evolving to Support Clinical and Regulatory Advancements?
Technological innovation in PRP tubes is focusing on optimizing platelet yield, enhancing separation efficiency, and meeting evolving clinical protocol needs. Multi-chamber tube designs now allow for built-in segregation of red blood cells, buffy coat, and plasma, minimizing manual handling post-spin. Advanced gel matrices within tubes are engineered to prevent platelet activation during separation, ensuring biologically potent concentrates. Some systems incorporate anticoagulants with pH stabilizers to maintain cellular integrity over extended processing times-particularly relevant in high-volume clinics or remote setups. Emerging trends include the development of photoprotective tubes for PRP formulations enriched with photosensitive biomolecules, as well as biodegradable plastic tubes that meet sustainability goals in hospital procurement. RFID-tagged caps and digitally traceable tube lots are being integrated into clinical systems to ensure procedural compliance, quality audits, and traceability. Automation-compatible tube formats that integrate with centrifuge racks and blood analyzers are reducing human error and supporting scalable deployment in multispecialty hospitals.From a regulatory standpoint, the standardization of PRP procedures by national health bodies and medical associations is influencing the demand for certified, single-use PRP tubes that conform to ISO 13485 and FDA/CE labeling norms. Vendors are also tailoring tube volumes (ranging from 5 ml to 30 ml) and labeling languages to cater to regional differences in therapeutic protocol and workflow. Additionally, the emergence of PRF (platelet rich fibrin) as an alternative is pushing tube manufacturers to diversify portfolios with silica-coated or titanium-lined tubes for fibrin matrix formation.
What Forces Are Propelling the Global Market for PRP Tubes Forward?
The growth in the platelet rich plasma tubes market is driven by several factors spanning clinical expansion, procedural affordability, and outpatient treatment trends. A key driver is the rising global demand for regenerative therapies as non-pharmacological alternatives in orthopedics, dermatology, and dental surgery. As these treatments shift from experimental to routine in many geographies, the demand for high-quality, pre-configured PRP tubes is intensifying-particularly among standalone clinics and ambulatory surgical centers. Minimally invasive aesthetics and dermatology are another strong driver, propelled by social media awareness and increased affordability of cosmetic procedures. PRP tubes tailored for facial rejuvenation and hair regrowth are in demand in regions like the Middle East, East Asia, and Latin America, where out-of-pocket procedures dominate. Moreover, the shift toward direct-to-clinic procurement, especially via e-commerce medical platforms, is simplifying access to global brands and expanding market reach for certified PRP tube systems.The growing emphasis on point-of-care treatments, combined with favorable reimbursement for PRP interventions in select countries, is pushing public and private providers to integrate PRP into mainstream care pathways. Additionally, technological convergence with portable centrifuges and mobile regenerative therapy kits is accelerating PRP access in rural or under-resourced settings. As PRP's clinical value becomes more widely validated and protocols get codified, demand for standardized, reliable PRP tubes is set to rise in both established and emerging healthcare markets.
Scope Of Study:
The report analyzes the Platelet Rich Plasma Tubes market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Usage (Skin Rejuvenation, Hair Restoration, Orthopedics Usage, Surgical Oncology, Gynecology); Volume (Below 5 ml Volume, 6 - 10 ml Volume, Above 10 ml Volume); End-Use (MedSpa End-Use, Dermatology Office End-Use, Hospitals & Healthcare Practice End-Use, Tricologist End-Use)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Skin Rejuvenation segment, which is expected to reach US$602.6 Million by 2030 with a CAGR of a 14.1%. The Hair Restoration segment is also set to grow at 13.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $236.2 Million in 2024, and China, forecasted to grow at an impressive 20.1% CAGR to reach $436.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Platelet Rich Plasma Tubes Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Platelet Rich Plasma Tubes Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Platelet Rich Plasma Tubes Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AdvaCare Pharma USA LLC, Arthrex, Bejing Hanbaihan Medical Devices (HBH), BS Medical Co. Ltd., Cangzhou Fukang Medical Supplies Co. Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Platelet Rich Plasma Tubes market report include:
- AdvaCare Pharma USA LLC
- Arthrex
- Bejing Hanbaihan Medical Devices (HBH)
- BS Medical Co. Ltd.
- Cangzhou Fukang Medical Supplies Co. Ltd.
- Emerging Medical Group LLC
- Henso Medical (Hangzhou) Co. Ltd.
- Integrity PRP
- Ippocare
- Juventix Regenerative Medical LLC
- Lingen Precision Medical Products Co. Ltd.
- Manso Medical Co. Ltd.
- Promed Solutions
- Rev-Med
- Rio Worldwide
- Selphyl (Aesthetic Factors)
- Terumo BCT
- Weigao Group
- Ybo Technologies Co. Ltd.
- Zhejiang Paulman
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AdvaCare Pharma USA LLC
- Arthrex
- Bejing Hanbaihan Medical Devices (HBH)
- BS Medical Co. Ltd.
- Cangzhou Fukang Medical Supplies Co. Ltd.
- Emerging Medical Group LLC
- Henso Medical (Hangzhou) Co. Ltd.
- Integrity PRP
- Ippocare
- Juventix Regenerative Medical LLC
- Lingen Precision Medical Products Co. Ltd.
- Manso Medical Co. Ltd.
- Promed Solutions
- Rev-Med
- Rio Worldwide
- Selphyl (Aesthetic Factors)
- Terumo BCT
- Weigao Group
- Ybo Technologies Co. Ltd.
- Zhejiang Paulman
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 376 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
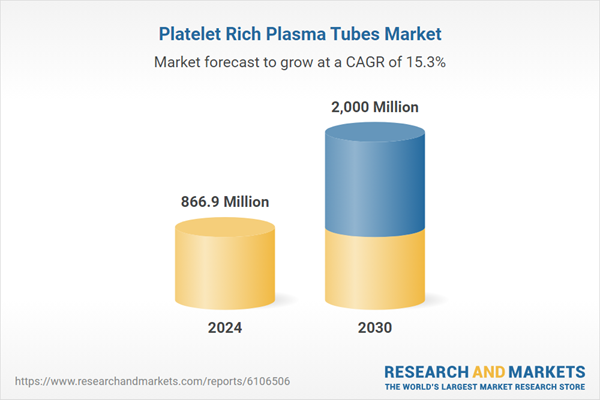
| Estimated Market Value in 2024 | 866.9 Million |
| Forecasted Market Value by 2030 | 2000 Million |
| Compound Annual Growth Rate | 15.3% |
| Regions Covered | Global |