Global Preclinical Respiration and Inhalation Lab Equipment Market - Key Trends & Drivers Summarized
How Is Technology Enhancing Precision and Throughput in Respiratory and Inhalation Studies?
Preclinical respiration and inhalation lab equipment is undergoing rapid technological refinement to improve measurement accuracy, exposure control, and biological modeling. Advances in plethysmography systems, whole-body exposure chambers, nose-only exposure towers, and aerosol generators are enabling researchers to simulate human inhalation scenarios with higher precision and consistency. These systems now integrate high-resolution pressure sensors, differential flow meters, and real-time data acquisition software that can monitor tidal volume, breathing rate, minute ventilation, and peak expiratory flow in conscious rodents or anesthetized subjects.Aerosol delivery systems are evolving with smart particle sizers, high-frequency ultrasonic nebulizers, and mass flow controllers that allow reproducible particle deposition across different regions of the respiratory tract. Novel systems also incorporate humidification and temperature regulation for physiologically relevant aerosol conditioning. Automated sampling and telemetry-enabled systems are being used to collect respiratory biomarkers, perform gas exchange measurements, and monitor respiratory depression or pulmonary irritation in response to drug candidates or environmental toxins. These technologies support both acute and chronic exposure protocols in preclinical pharmacokinetics, toxicology, and inhaled therapeutics development pipelines.
Which Research Domains Are Expanding the Use of Preclinical Respiratory Equipment?
While preclinical respiration equipment has long been used in pulmonary drug delivery studies, its applications are expanding across several interdisciplinary domains. In respiratory medicine R&D, these systems are central to evaluating efficacy and safety of inhaled corticosteroids, bronchodilators, and novel biologics for asthma, COPD, cystic fibrosis, and idiopathic pulmonary fibrosis. In oncology, inhaled formulations for chemotherapeutic delivery are being tested for site-specific deposition in lung tumors, supported by intratracheal and intranasal dosing rigs.The equipment is also being used in environmental health studies to assess inhalation toxicity from air pollutants, nanoparticles, and industrial chemicals. Regulatory bodies such as OECD and U.S. EPA are increasingly emphasizing inhalation exposure studies in their guidelines for chemical risk assessment. Additionally, infectious disease researchers are employing nose-only exposure chambers and aerosolized pathogen systems to evaluate transmission and respiratory effects of airborne viruses and bacteria, including SARS-CoV-2, influenza, and tuberculosis. The integration of real-time respiratory telemetry with animal models in biosafety level (BSL) environments is enhancing the resolution of data on immune response, lung pathology, and dose-response kinetics in infectious models.
How Are Regulatory and Ethical Standards Driving Technological Innovation and Compliance?
Stringent regulatory frameworks and evolving animal welfare standards are influencing the design and deployment of preclinical respiratory and inhalation systems. Agencies such as the FDA, EMA, and OECD require detailed characterization of inhaled drug delivery parameters, including particle size distribution, delivered dose, and deposition profile. As a result, preclinical equipment manufacturers are investing in validation-ready systems with traceable calibration, automated dosing validation, and GLP-compliant software platforms. Aerosol exposure systems are now engineered to support real-time feedback control for consistent dose delivery over extended experimental periods.Animal welfare regulations are also pushing equipment developers to reduce animal stress and refine exposure protocols. Nose-only systems are being redesigned with ergonomic restraints and minimal airflow turbulence to reduce discomfort. Whole-body chambers now include partitioned airflow zones and environmental enrichment features. Furthermore, the 3Rs principle (Replacement, Reduction, Refinement) is encouraging multi-animal telemetry systems and multiplexed plethysmographs to improve data throughput while minimizing the number of animals required. This convergence of regulatory compliance and ethical design is driving a new generation of respiratory and inhalation platforms tailored for translational research and preclinical validation.
What Is Accelerating Market Growth for Preclinical Respiration and Inhalation Lab Equipment?
The growth in the global preclinical respiration and inhalation lab equipment market is driven by rising demand for targeted pulmonary therapeutics, regulatory mandates for inhalation toxicology, and global investment in respiratory research infrastructure. The surge in chronic respiratory conditions and respiratory infections-compounded by air pollution, smoking, and climate change-is pushing pharmaceutical and biotech firms to prioritize inhaled drug formulations, driving demand for preclinical evaluation tools. Post-COVID R&D investments in inhaled antivirals and vaccines have further accelerated this trend.Governments and academic institutions are expanding laboratory capacity for aerosol toxicology, bioaerosol containment, and respiratory disease modeling, especially in North America, Europe, and East Asia. Inhalation drug developers are collaborating with CROs to validate delivery systems and optimize dosing strategies using advanced equipment. Equipment manufacturers are also bundling hardware with software and analytics platforms that allow researchers to simulate human pharmacokinetics using computational fluid dynamics (CFD) and physiologically based pharmacokinetic (PBPK) models.
Key players such as SCIREQ, Buxco/DSI (Harvard Bioscience), CH Technologies, InExpose, and Kent Scientific are expanding their product lines to include integrated inhalation platforms, modular exposure towers, and AI-powered analytics suites. With rising regulatory scrutiny, drug pipeline diversification, and the growing prevalence of respiratory health challenges, the preclinical respiration and inhalation equipment market is set to witness steady growth across pharmaceutical, environmental, and academic research sectors.
Scope Of Study:
The report analyzes the Preclinical Respiration and Inhalation Lab Equipment market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Type (Respiration Equipment, Inhalation Equipment); End-Use (CROs & Academic Institutes End-Use, Pharma & Biotech Companies End-Use)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Respiration Equipment segment, which is expected to reach US$18.2 Million by 2030 with a CAGR of a 4.9%. The Inhalation Equipment segment is also set to grow at 2.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $5.3 Million in 2024, and China, forecasted to grow at an impressive 4.2% CAGR to reach $4.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Preclinical Respiration and Inhalation Lab Equipment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Preclinical Respiration and Inhalation Lab Equipment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Preclinical Respiration and Inhalation Lab Equipment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ADInstruments, Aeroqual Ltd, Buxco Research Systems (DSI), Charles River Laboratories, CH Technologies (USA), Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Preclinical Respiration and Inhalation Lab Equipment market report include:
- ADInstruments
- Aeroqual Ltd
- Buxco Research Systems (DSI)
- Charles River Laboratories
- CH Technologies (USA), Inc.
- Emka TECHNOLOGIES
- Envigo (now part of Inotiv)
- Harvard Apparatus
- InExpose (Scireq)
- Kent Scientific Corporation
- La Jolla Alcohol Research, Inc.
- LabLogic Systems Ltd
- Med Associates Inc.
- MGC Diagnostics Corporation
- NoseOnly Research
- PHYSIO-TECH Co., Ltd.
- SciReq Inc. (Scientific Respiratory Equipment)
- Somni Scientific
- TSE Systems GmbH
- VetEquip Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ADInstruments
- Aeroqual Ltd
- Buxco Research Systems (DSI)
- Charles River Laboratories
- CH Technologies (USA), Inc.
- Emka TECHNOLOGIES
- Envigo (now part of Inotiv)
- Harvard Apparatus
- InExpose (Scireq)
- Kent Scientific Corporation
- La Jolla Alcohol Research, Inc.
- LabLogic Systems Ltd
- Med Associates Inc.
- MGC Diagnostics Corporation
- NoseOnly Research
- PHYSIO-TECH Co., Ltd.
- SciReq Inc. (Scientific Respiratory Equipment)
- Somni Scientific
- TSE Systems GmbH
- VetEquip Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 170 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
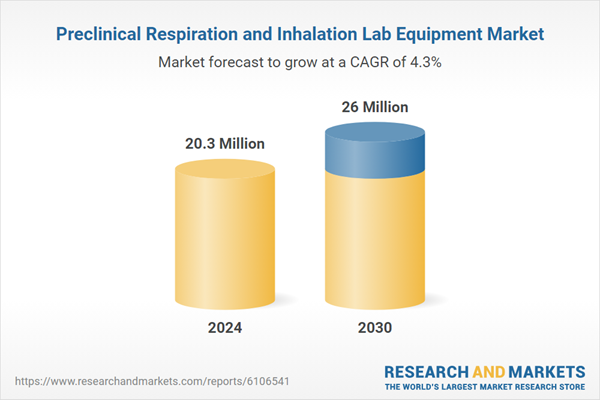
| Estimated Market Value in 2024 | 20.3 Million |
| Forecasted Market Value by 2030 | 26 Million |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |