Global Preoperative Infection Prevention and Wound Cleansing Devices Market - Key Trends & Drivers Summarized
How Are Preoperative Infection Control Protocols Shaping Demand for Advanced Cleansing Devices?
Preoperative infection prevention has become a critical focal point in surgical care pathways due to the growing emphasis on reducing surgical site infections (SSIs)-one of the most common healthcare-associated infections (HAIs). Healthcare systems are investing in advanced wound cleansing devices that deliver consistent, antiseptic-infused lavage or debridement in preoperative settings. These devices, ranging from pressurized saline irrigators to automated cleansing systems, are being designed to standardize preoperative skin and wound preparation, improve sterility margins, and reduce microbial colonization at surgical entry points.Solutions containing chlorhexidine gluconate (CHG), povidone-iodine, and octenidine are frequently used in conjunction with these devices to offer broad-spectrum antimicrobial coverage. The use of pulsed lavage and low-pressure irrigation devices has expanded from orthopedic surgeries to general and laparoscopic procedures, driven by their ability to reduce bioburden in contaminated and chronic wounds. Additionally, advanced hydrodebridement systems with integrated suction are gaining adoption in cases where preoperative cleansing is performed on traumatic wounds or pressure ulcers to reduce microbial load before surgical intervention. The shift from manual to automated and semi-automated devices reflects the need for consistency, time efficiency, and reduced clinician burden.
In Which Clinical Settings and Specialties Are Preoperative Cleansing Devices Making the Most Impact?
Preoperative infection prevention devices are increasingly deployed across surgical wards, ambulatory surgical centers (ASCs), emergency departments, and trauma stabilization units. Orthopedic and joint replacement surgeries-particularly hip and knee arthroplasties-have seen widespread adoption of automated wound cleansing systems due to high SSI risk and associated readmission costs. In cardiothoracic and neurosurgical procedures, where infection can lead to catastrophic complications, preoperative antiseptic protocols are often reinforced with specialized cleansing tools to achieve maximum site sterility.Burn care units and trauma centers are leveraging wound cleansing devices to manage acute injuries, often as a bridge to reconstructive surgery. Diabetic foot ulcer management protocols also include cleansing devices to prepare infected or necrotic tissue for surgical debridement. In outpatient surgery centers, preoperative skin cleansing devices are being introduced to minimize cross-contamination, streamline workflow, and comply with infection control mandates. Furthermore, military medical facilities and disaster relief teams are adopting portable, battery-operated cleansing units for field surgeries, enabling sterile preparation in austere conditions. This broadening clinical footprint is reinforcing the role of these devices in surgical infection mitigation across a range of specialties.
How Are Guidelines, Technological Improvements, and Cost Metrics Influencing Market Growth?
Regulatory and clinical guidelines from bodies such as the CDC, WHO, and NICE are playing a significant role in standardizing preoperative antiseptic protocols. These recommendations underscore the importance of skin and wound cleansing as a first-line defense against SSIs. Hospitals are increasingly required to report infection rates and adhere to infection control standards for accreditation and reimbursement-driving demand for standardized, validated cleansing technologies. Bundled payments and value-based care models are further motivating health systems to invest in solutions that reduce surgical complications and length of hospital stays.Technology developers are responding with ergonomic, single-use, and sensor-enabled cleansing devices that reduce contamination risk and simplify compliance tracking. Some newer systems integrate antimicrobial delivery with feedback sensors that guide optimal contact time or coverage area. Additionally, closed-system designs with built-in fluid reservoirs and suction functions are gaining popularity for reducing splash exposure and handling errors. Cost-effectiveness remains a key purchasing criterion, especially for high-volume facilities and public health systems. Manufacturers are offering device kits bundled with antiseptic solutions and disposable components to provide all-in-one infection control solutions that improve adherence and reduce setup time.
What Factors Are Driving the Expansion of the Global Market for Preoperative Cleansing Devices?
The growth in the global preoperative infection prevention and wound cleansing devices market is driven by rising surgical volumes, increased awareness of HAIs, and pressure on hospitals to reduce postoperative complications. As the global population ages and chronic disease prevalence rises, the number of surgical interventions-including orthopedic, cardiovascular, and gastrointestinal procedures-is increasing sharply. With every additional surgery comes higher risk of SSIs, particularly in immunocompromised or diabetic patients, reinforcing the clinical need for effective preoperative cleansing protocols.The COVID-19 pandemic has further heightened infection control awareness, prompting health systems to reassess perioperative practices and invest in single-use, touchless, and automated infection prevention tools. The recovery of elective surgeries and expansion of ASC networks in North America, Europe, and parts of Asia-Pacific are creating demand for compact, rapid-deployment cleansing solutions. Emerging economies are witnessing greater adoption due to international aid programs, rising healthcare infrastructure, and training initiatives aimed at improving surgical outcomes.
Leading manufacturers such as 3M, Mölnlycke, Smith & Nephew, and Irrimax are focusing on portfolio diversification, clinical evidence generation, and regional distribution expansion. Product development is increasingly centered around user-friendly interfaces, cross-specialty applicability, and antimicrobial efficacy. With regulatory alignment, technological innovation, and cost containment shaping procurement decisions, the market for preoperative wound cleansing devices is poised for consistent growth, especially in settings striving for surgical safety excellence and zero-SSI targets.
Scope Of Study:
The report analyzes the Preoperative Infection Prevention and Wound Cleansing Devices market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Product (Preoperative Infection Prevention Devices, Preoperative Wound Cleansing Devices); Surgery (Cataract Surgery, Cesarean Surgery, Appendectomy Surgery, Colectomy & Colostomy Surgery, Esophagectomy Surgery, Biopsy Surgery, Cholecystectomy Surgery, Other Surgeries); Application (Preoperative Hair Removal Application, Preoperative Skin Preparation Application, Intraoperative Wound Irrigation Solution Application)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Preoperative Infection Prevention Devices segment, which is expected to reach US$2.4 Billion by 2030 with a CAGR of a 6.7%. The Preoperative Wound Cleansing Devices segment is also set to grow at 3.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $638.8 Million in 2024, and China, forecasted to grow at an impressive 9.3% CAGR to reach $669.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Preoperative Infection Prevention and Wound Cleansing Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Preoperative Infection Prevention and Wound Cleansing Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Preoperative Infection Prevention and Wound Cleansing Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M Health Care, Advanced Sterilization Products (ASP), B. Braun Melsungen AG, Baxter International Inc., BD (Becton, Dickinson and Company) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Preoperative Infection Prevention and Wound Cleansing Devices market report include:
- 3M Health Care
- Advanced Sterilization Products (ASP)
- B. Braun Melsungen AG
- Baxter International Inc.
- BD (Becton, Dickinson and Company)
- Cardinal Health, Inc.
- CareFusion (part of BD)
- Coloplast A/S
- ConvaTec Group plc
- Ecolab Inc.
- Ethicon (Johnson & Johnson MedTech)
- GAMA Healthcare Ltd
- HARTMANN Group
- Medela AG
- Medline Industries, LP
- Mölnlycke Health Care
- Paul Hartmann AG
- Schülke & Mayr GmbH
- Smith & Nephew plc
- Stryker Corporation
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M Health Care
- Advanced Sterilization Products (ASP)
- B. Braun Melsungen AG
- Baxter International Inc.
- BD (Becton, Dickinson and Company)
- Cardinal Health, Inc.
- CareFusion (part of BD)
- Coloplast A/S
- ConvaTec Group plc
- Ecolab Inc.
- Ethicon (Johnson & Johnson MedTech)
- GAMA Healthcare Ltd
- HARTMANN Group
- Medela AG
- Medline Industries, LP
- Mölnlycke Health Care
- Paul Hartmann AG
- Schülke & Mayr GmbH
- Smith & Nephew plc
- Stryker Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 386 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
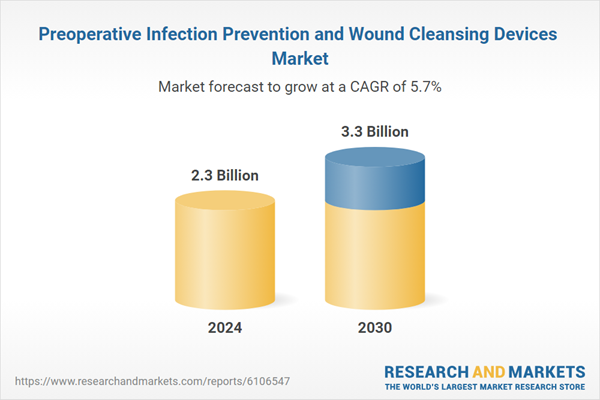
| Estimated Market Value in 2024 | 2.3 Billion |
| Forecasted Market Value by 2030 | 3.3 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |