Global Intracranial Therapeutic Delivery Market - Key Trends & Drivers Summarized
Why Is Intracranial Therapeutic Delivery Emerging as a Critical Innovation in Neuroscience?
Intracranial therapeutic delivery is rapidly gaining prominence in the field of neuroscience as researchers and clinicians confront the formidable challenge of treating complex brain disorders that are often inaccessible or unresponsive to conventional systemic therapies. The blood-brain barrier (BBB), which protects the central nervous system from toxins and pathogens, also prevents many potentially beneficial drugs from reaching therapeutic concentrations in brain tissue. Intracranial delivery techniques, which bypass or penetrate the BBB, are therefore revolutionizing the treatment of neurological conditions by allowing for localized, controlled, and direct administration of therapeutic agents to specific brain regions. These techniques are critical in managing diseases such as glioblastoma, Parkinson's disease, epilepsy, Alzheimer's, and rare pediatric neurodegenerative disorders. Methods such as convection-enhanced delivery, intrathecal and intraventricular infusions, and implantable drug delivery systems are enabling clinicians to achieve higher efficacy with lower systemic toxicity. They also permit the use of novel treatment modalities like gene therapy, monoclonal antibodies, and nanoparticles that require targeted delivery to be effective. As the burden of neurological diseases continues to rise globally and current treatment approaches fall short in halting or reversing disease progression, intracranial therapeutic delivery is emerging not just as an option but as a necessity for transforming outcomes in neuro-oncology, neurodegeneration, and beyond.How Is Technology Enhancing the Precision and Safety of Intracranial Therapeutic Techniques?
The evolution of intracranial therapeutic delivery is being propelled by a wave of technological advancements that are significantly improving the precision, control, and safety of treatment. Real-time imaging technologies such as MRI-guided systems and neuronavigation platforms allow for highly accurate placement of catheters and implants, minimizing damage to healthy brain tissue and improving patient outcomes. Innovations in biomaterials have led to the development of biodegradable polymers and hydrogels that can deliver drugs over sustained periods directly within the brain. These materials can be engineered to release therapeutic agents in response to specific triggers like pH or temperature, offering dynamic control over drug concentration and timing. Microelectromechanical systems (MEMS) and nanotechnology are enabling the creation of miniaturized pumps and valves that can modulate flow rates and respond to physiological signals. Additionally, researchers are integrating wireless control mechanisms into implantable devices, allowing clinicians to remotely adjust dosing or switch therapies based on patient response. Advances in pharmacogenomics and molecular diagnostics are also playing a key role, as they help personalize therapy by aligning drug choice and delivery methods with the patient's genetic and molecular profile. Techniques such as focused ultrasound are being studied for their potential to temporarily open the blood-brain barrier, allowing noninvasive delivery of systemically administered drugs to targeted brain areas. Collectively, these innovations are making intracranial therapeutic delivery not only more effective but also safer and more adaptable, opening new possibilities in the management of previously intractable neurological conditions.How Do Disease Complexity and Patient-Specific Needs Shape Intracranial Delivery Strategies?
Intracranial therapeutic delivery strategies are heavily influenced by the type and location of the neurological disease, its stage of progression, and the unique anatomical and physiological characteristics of each patient. In cases such as high-grade gliomas or brain metastases, where tumors infiltrate surrounding tissue and are resistant to systemic chemotherapy, direct infusion of cytotoxic agents or immunotherapeutics into the tumor bed provides a more targeted approach with fewer systemic side effects. Neurodegenerative conditions like Parkinson's disease require delivery to deep brain structures such as the substantia nigra or basal ganglia, necessitating high spatial accuracy and chronic administration capabilities. In pediatric patients with lysosomal storage disorders or leukodystrophies, intracranial gene therapy administered via viral vectors into the cerebrospinal fluid or brain ventricles offers the potential for long-term correction of underlying genetic defects. Each condition poses unique challenges in terms of delivery route, formulation stability, and dosing schedule. Furthermore, patient factors such as age, previous surgeries, immune response, and coexisting medical conditions also shape the design and implementation of intracranial delivery protocols. Hospitals and research centers are now leveraging multidisciplinary teams of neurosurgeons, neurologists, radiologists, and pharmacologists to tailor treatment plans that optimize therapeutic efficacy while minimizing procedural risks. There is also growing interest in hybrid approaches that combine intracranial and systemic delivery to target both localized lesions and diffuse pathology. As precision medicine continues to gain traction, the ability to individualize intracranial therapeutic delivery will be crucial in improving treatment outcomes and expanding the reach of advanced neurotherapeutics.What Factors Are Driving the Global Expansion of the Intracranial Therapeutic Delivery Market?
The growth in the intracranial therapeutic delivery market is driven by several intersecting trends related to disease burden, technological capability, regulatory support, and evolving treatment paradigms. One of the major drivers is the increasing global incidence of neurological disorders, particularly in aging populations, where conditions such as Alzheimer's, Parkinson's, and stroke are on the rise. The limited efficacy of existing treatment modalities for these conditions is prompting greater investment in targeted, localized therapies that can overcome barriers like the BBB and disease heterogeneity. The expanding pipeline of biologics, gene therapies, and cell-based treatments that require direct brain administration is also boosting demand for innovative delivery platforms. Regulatory agencies are providing fast-track and orphan drug designations to companies developing novel neurological therapies, accelerating the development and approval of intracranial delivery technologies. Significant funding from public and private sources is supporting clinical trials that explore the use of intracranial delivery for both rare and common CNS diseases. Meanwhile, technological advancements in imaging, navigation, and biomaterials are reducing procedural risk and enhancing physician confidence in these techniques. Increasing collaboration between academic research centers, biotech firms, and medical device manufacturers is leading to integrated solutions that combine diagnostics, delivery, and therapeutic monitoring into a single platform. Furthermore, patient advocacy groups and neurological foundations are raising awareness and pushing for earlier diagnosis and more aggressive treatment approaches, creating demand for therapies that can intervene directly at the site of pathology. These combined forces are accelerating the global adoption of intracranial therapeutic delivery and positioning it as a cornerstone of future neuroscience innovation.Report Scope
The report analyzes the Intracranial Therapeutic Delivery market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Therapy (Cell-based Therapy, Gene Therapy, Enzyme Replacement Therapy); Indication (Spinal Muscular Atrophy Indication, Multiple Sclerosis Indication, Batten Disease Indication, Amyotrophic Lateral Sclerosis Indication).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cell-based Therapy segment, which is expected to reach US$1.8 Billion by 2030 with a CAGR of a 8.2%. The Gene Therapy segment is also set to grow at 6.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $584.7 Million in 2024, and China, forecasted to grow at an impressive 11.4% CAGR to reach $688.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Intracranial Therapeutic Delivery Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Intracranial Therapeutic Delivery Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Intracranial Therapeutic Delivery Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Beiersdorf AG, Bodywise (UK) Ltd, Cora, Edgewell Personal Care, Essity AB and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Intracranial Therapeutic Delivery market report include:
- Abyrx, Inc.
- Alcyone Therapeutics
- Antisense Therapeutics Ltd
- B. Braun Melsungen AG
- Biogen Inc.
- Brainlab AG
- Cala Health, Inc.
- Cerebrum Tech
- Codman Neuro (Integra LifeSciences)
- Elekta AB
- Flowonix Medical Inc.
- Insightec Ltd.
- Medtronic plc
- NeuroSigma, Inc.
- Neurotech Pharmaceuticals, Inc.
- NXDC, Inc. (Neuralink competitor)
- Roche Holding AG
- Renishaw plc
- Synchron, Inc.
- VeriTouch Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abyrx, Inc.
- Alcyone Therapeutics
- Antisense Therapeutics Ltd
- B. Braun Melsungen AG
- Biogen Inc.
- Brainlab AG
- Cala Health, Inc.
- Cerebrum Tech
- Codman Neuro (Integra LifeSciences)
- Elekta AB
- Flowonix Medical Inc.
- Insightec Ltd.
- Medtronic plc
- NeuroSigma, Inc.
- Neurotech Pharmaceuticals, Inc.
- NXDC, Inc. (Neuralink competitor)
- Roche Holding AG
- Renishaw plc
- Synchron, Inc.
- VeriTouch Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 272 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
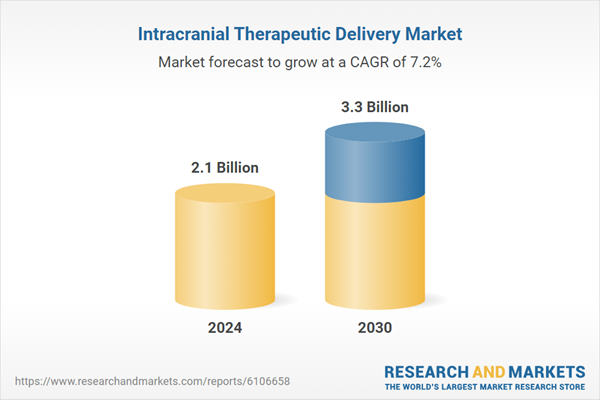
| Estimated Market Value ( USD | $ 2.1 Billion |
| Forecasted Market Value ( USD | $ 3.3 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |