Global Intravenous Therapy and Vein Access Market - Key Trends & Drivers Summarized
Why Is Intravenous Therapy and Vein Access a Cornerstone of Modern Clinical Care?
Intravenous therapy and vein access have become fundamental to contemporary healthcare, enabling rapid and efficient administration of fluids, medications, nutrients, and blood products directly into the bloodstream. This mode of delivery offers immediate bioavailability, making it indispensable for critical care, emergency medicine, surgery, and chronic disease management. The versatility of intravenous (IV) therapy supports a wide range of applications, from administering antibiotics and chemotherapy to maintaining hydration and correcting electrolyte imbalances. In hospitals, it is often the first line of intervention for acute illnesses and trauma, while in outpatient and home care settings, it supports long-term treatments for conditions such as cancer, autoimmune diseases, and infections requiring prolonged antimicrobial therapy. Vein access is essential for drawing blood, delivering diagnostics, and performing therapeutic apheresis, making it integral to both preventive and curative care. The growing prevalence of lifestyle-related chronic illnesses, such as diabetes, cardiovascular disease, and kidney failure, has significantly increased the need for reliable vascular access and ongoing IV treatment. In pediatric and geriatric populations, specialized vein access devices improve patient safety and comfort while reducing procedural complications. With healthcare systems increasingly emphasizing efficiency and outcomes, intravenous therapy has established itself as a critical clinical tool, underpinning both emergency interventions and structured treatment pathways across all levels of care.How Are Innovations Improving the Safety and Precision of Vein Access and Infusion Therapy?
Technological advances are revolutionizing intravenous therapy and vein access by enhancing precision, minimizing complications, and making procedures more patient-friendly. One of the most notable developments is the use of ultrasound-guided insertion techniques, which have dramatically improved first-attempt success rates, especially in patients with difficult venous access. Vein visualization devices using infrared light and near-infrared imaging allow clinicians to identify optimal veins without repeated punctures, reducing discomfort and risk of infection. Smart infusion pumps equipped with dose error reduction software and barcode scanning are becoming standard in hospitals, helping to prevent medication errors and ensure accurate flow rates. Catheter materials have evolved to include antimicrobial coatings and biocompatible polymers that lower the risk of thrombosis and catheter-associated bloodstream infections. Central venous catheters, peripherally inserted central catheters (PICCs), and midline catheters are now available in multiple configurations to meet the specific requirements of different therapies and durations. Needle-free connectors and closed-system transfer devices are also reducing the risk of needle-stick injuries and contamination. Meanwhile, wearable infusion devices are enabling ambulatory and home-based IV therapy, giving patients greater flexibility and comfort. Integration with electronic medical records allows for continuous monitoring and documentation of infusion parameters, supporting evidence-based decision-making and better clinical outcomes. These innovations are collectively reshaping the administration of intravenous therapy by making it safer, more accurate, and more accessible across diverse patient populations.How Do Clinical Settings and Patient Profiles Influence the Selection of IV Access Devices?
The choice of intravenous access devices and infusion strategies is closely aligned with the clinical setting, duration of therapy, and individual patient characteristics. In emergency rooms and intensive care units, peripheral IV lines are often placed quickly for immediate fluid and drug delivery, with central venous access considered for more complex or long-term treatments. Surgical patients may require short-term access for anesthesia and postoperative recovery, while oncology patients benefit from implantable ports or PICC lines to facilitate repetitive chemotherapy infusions with minimal discomfort. Neonates and pediatric patients present unique challenges due to smaller vein size and increased sensitivity, leading to the use of specialized microcatheters and pain-reduction techniques. For patients with chronic conditions requiring frequent or continuous infusions, such as those with cystic fibrosis or immune deficiencies, durable and low-maintenance devices are prioritized. Patient comorbidities also affect device selection, with those at high risk of infection or thrombosis requiring antimicrobial or heparin-bonded catheters. Home healthcare settings demand user-friendly and portable solutions that support patient independence while ensuring clinical oversight. In resource-limited settings, the availability of skilled personnel and equipment may dictate the use of simpler, lower-cost options, although efforts are being made globally to standardize protocols and train healthcare workers in advanced techniques. The diversity in clinical applications and patient needs continues to drive product innovation and the refinement of insertion and maintenance protocols, underscoring the complexity and importance of tailored vein access solutions within the broader spectrum of intravenous therapy.What Is Driving the Expansion of the Global Intravenous Therapy and Vein Access Market?
The growth in the intravenous therapy and vein access market is driven by multiple factors rooted in demographic shifts, rising disease burden, healthcare modernization, and ongoing advancements in medical devices. One of the primary drivers is the global increase in chronic and infectious diseases that require prolonged or intensive intravenous treatment, such as cancer, sepsis, and autoimmune disorders. The aging population, particularly in developed countries, is also contributing to increased demand, as older adults often require more frequent hospitalizations and complex medication regimens. The expansion of outpatient and home-based care services is fostering the need for portable and patient-friendly infusion technologies that can be used safely outside hospital settings. Technological improvements in catheter design, drug delivery systems, and infusion monitoring are encouraging more clinicians to adopt sophisticated and safer IV solutions. Additionally, global efforts to reduce hospital-acquired infections are leading to widespread adoption of closed-system devices and antimicrobial materials. In emerging economies, growing investment in healthcare infrastructure and rising awareness of best practices in IV therapy are opening new markets and expanding access to advanced vein access techniques. Training programs and certification standards are also improving practitioner skill levels, reducing procedural errors, and boosting patient outcomes. Furthermore, regulatory approvals for new biologics and intravenous medications are creating a larger pool of therapies that depend on reliable infusion delivery. As healthcare systems worldwide continue to emphasize cost-effectiveness, safety, and patient-centered care, the market for intravenous therapy and vein access is poised for sustained growth across both hospital and non-hospital settings.Report Scope
The report analyzes the Intravenous Therapy and Vein Access market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Medication Administration Application, Blood-based Products Application, Nutrition & Buffer Solution Application, Volume Expander Application); End-Use (Hospitals End-Use, Clinics End-Use, Ambulatory Surgery Centers End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Medication Administration Application segment, which is expected to reach US$12.2 Billion by 2030 with a CAGR of a 2.5%. The Blood-based Products Application segment is also set to grow at 4.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $7.1 Billion in 2024, and China, forecasted to grow at an impressive 5.9% CAGR to reach $6.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Intravenous Therapy and Vein Access Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Intravenous Therapy and Vein Access Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Intravenous Therapy and Vein Access Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abiomed, Inc., Arrow International, Inc. (Teleflex), BIOTRONIK SE & Co. KG, Boston Scientific Corporation, Cardiovascular Systems Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Intravenous Therapy and Vein Access market report include:
- Ajinomoto Health & Nutrition North America, Inc.
- AngioDynamics, Inc.
- B. Braun Medical Inc.
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company (BD)
- Cardinal Health
- Core IV Therapy, LLC
- Cryojuvenate UK Ltd.
- CSL Vifor
- Drip Hydration
- Fresenius Kabi
- Haemonetics Corporation
- ICU Medical, Inc.
- Medtronic plc
- Otsuka Pharmaceutical Co., Ltd.
- Polymedicure
- Smiths Medical, Inc.
- Teleflex Incorporated
- Terumo Corporation
- Vygon SAS
- Vetter Pharma-Fertigung GmbH & Co. KG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ajinomoto Health & Nutrition North America, Inc.
- AngioDynamics, Inc.
- B. Braun Medical Inc.
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company (BD)
- Cardinal Health
- Core IV Therapy, LLC
- Cryojuvenate UK Ltd.
- CSL Vifor
- Drip Hydration
- Fresenius Kabi
- Haemonetics Corporation
- ICU Medical, Inc.
- Medtronic plc
- Otsuka Pharmaceutical Co., Ltd.
- Polymedicure
- Smiths Medical, Inc.
- Teleflex Incorporated
- Terumo Corporation
- Vygon SAS
- Vetter Pharma-Fertigung GmbH & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
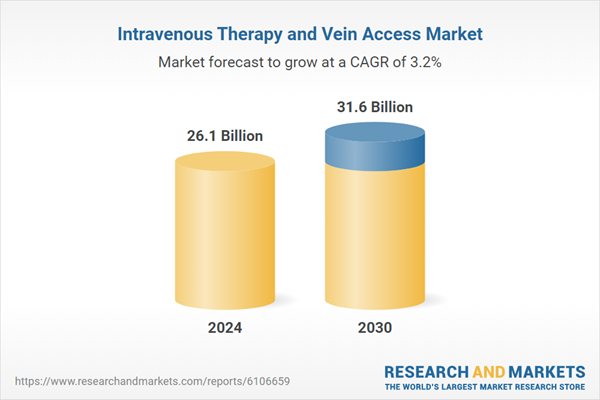
| No. of Pages | 284 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 26.1 Billion |
| Forecasted Market Value ( USD | $ 31.6 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |