Global In-Vitro Toxicology Assays Market - Key Trends & Drivers Summarized
Why Are In-Vitro Toxicology Assays Becoming Central to Modern Safety Testing?
In-vitro toxicology assays have emerged as a vital component of contemporary safety assessment strategies, largely due to their ability to provide detailed insights into the toxicological effects of chemical substances, pharmaceuticals, and consumer products without the ethical and practical challenges associated with animal testing. These assays use cultured cells, tissues, or reconstructed human biological systems to model specific organ functions, allowing researchers to assess cytotoxicity, genotoxicity, oxidative stress, and other cellular responses in a controlled laboratory setting. The growing demand for alternative testing models is being driven by stricter regulatory frameworks, such as the European Union's ban on animal testing for cosmetics and global movements advocating for the reduction of animal use in research. In-vitro assays offer faster turnaround, lower costs, and increased scalability compared to in-vivo models, making them attractive to pharmaceutical companies, chemical manufacturers, and regulatory bodies alike. Their ability to screen thousands of compounds rapidly using high-throughput platforms also accelerates the drug development process and supports chemical safety evaluation at an industrial scale. Additionally, these assays enable a deeper understanding of dose-response relationships and mechanisms of action by isolating specific molecular pathways. As the complexity of manufactured substances grows and safety regulations tighten, in-vitro toxicology is becoming indispensable in early-stage screening, risk assessment, and regulatory compliance efforts across a variety of industries.How Is Technology Advancing the Accuracy and Utility of In-Vitro Toxicology Methods?
Technological innovation is revolutionizing the performance and applicability of in-vitro toxicology assays by enhancing their biological relevance, sensitivity, and predictive power. One major advancement is the use of 3D cell cultures and organ-on-chip systems that mimic the structural and functional complexity of human tissues more accurately than traditional two-dimensional models. These advanced platforms enable the study of tissue-specific responses and long-term toxicity effects, providing a more realistic assessment of how substances interact with human biology. Integration with microfluidics allows for dynamic flow conditions that replicate physiological environments, further improving assay precision. High-content screening technologies are making it possible to analyze multiple cellular parameters simultaneously, such as morphology, viability, and protein expression, through automated imaging and data analytics. Artificial intelligence and machine learning algorithms are being deployed to process vast amounts of assay data, identify toxicity patterns, and predict outcomes with increasing accuracy. Additionally, the incorporation of omics technologies, including genomics, transcriptomics, and metabolomics, enables mechanistic toxicology studies that uncover pathway-level insights and support biomarker discovery. Improvements in assay standardization and validation are also facilitating regulatory acceptance, with frameworks now in place to assess reproducibility and relevance across laboratories and geographies. These technological leaps are not only expanding the scientific capabilities of in-vitro toxicology but also reinforcing its role as a cornerstone of modern toxicological assessment and product development.How Are Regulatory and Industry Demands Shaping the In-Vitro Toxicology Assay Landscape?
The growing influence of regulatory policies and industry needs is significantly shaping the direction and adoption of in-vitro toxicology assays. Governments and regulatory agencies across the globe are increasingly requiring non-animal testing data for chemical registration, drug approval, and product safety evaluation. Agencies such as the European Medicines Agency (EMA), the U.S. Environmental Protection Agency (EPA), and the Organization for Economic Cooperation and Development (OECD) are actively endorsing validated in-vitro test guidelines, which are helping to build trust in the scientific community and streamline approval processes. Industry players, especially in pharmaceuticals, cosmetics, and agrochemicals, are responding to these shifts by investing in assay development, automation platforms, and integrated data systems that align with regulatory expectations. The increased public scrutiny surrounding animal welfare is also pushing corporations to adopt more transparent and ethical testing practices. Moreover, the drive toward personalized medicine is requiring more precise toxicity screening tools that account for individual variability, further strengthening the case for human-relevant in-vitro models. Startups and research organizations are collaborating with regulatory bodies to co-develop standards that ensure assay consistency and interoperability across jurisdictions. In many sectors, in-vitro toxicology is now being incorporated early in the product development pipeline to reduce late-stage failures, enhance safety profiles, and meet environmental health and safety benchmarks. These intersecting pressures from regulators, consumers, and scientific advancements are reshaping how companies approach safety testing, cementing in-vitro toxicology assays as essential tools for responsible innovation and global compliance.What Is Fueling the Global Growth of the In-Vitro Toxicology Assays Market?
The growth in the in-vitro toxicology assays market is driven by a combination of regulatory reform, scientific advancement, industrial demand, and societal expectations for more ethical and effective testing methods. One of the key growth drivers is the global momentum toward reducing or eliminating animal testing, particularly in cosmetics and consumer health products, where public pressure and legislation are prompting companies to shift toward validated in-vitro alternatives. Simultaneously, the pharmaceutical and biotechnology sectors are increasingly using in-vitro assays to de-risk early-stage development and screen vast chemical libraries more cost-effectively and rapidly. The rise in environmental toxicity concerns and chemical exposure monitoring is also expanding demand for in-vitro testing in industrial and governmental contexts. Technological breakthroughs in cell modeling, artificial intelligence, and data integration are further enabling more accurate, scalable, and reproducible testing platforms that meet both scientific and regulatory standards. In emerging economies, increased investment in research infrastructure, rising pharmaceutical R&D spending, and improvements in laboratory capacity are contributing to the broader adoption of in-vitro testing technologies. Furthermore, collaborations between academia, industry, and public health agencies are fostering the development of next-generation toxicology models and platforms that can address complex endpoints such as endocrine disruption and chronic toxicity. As the intersection of technology, policy, and ethics continues to evolve, the global in-vitro toxicology assays market is poised for continued expansion, offering safer, faster, and more human-relevant testing methods for a diverse range of industries and applications.Report Scope
The report analyzes the In-Vitro Toxicology Assays market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Method (Cellular Assay Method, Live Cells Method, Fixed Cells Method, Other Methods); Test Type (Cannabis Testing Type, Nicotine Testing Type); Technology (3D Cell Culture Technology, Mass Spectrometry Technology, Flow Cytometry Technology, Other Technologies); Application (Genetic Toxicity Testing Application, Carcinogenicity Testing Application, Cytotoxicity Testing Application, Mutagenicity Testing Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cellular Assay Method segment, which is expected to reach US$2.3 Billion by 2030 with a CAGR of a 18.3%. The Live Cells Method segment is also set to grow at 13.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $586.6 Million in 2024, and China, forecasted to grow at an impressive 21.4% CAGR to reach $1.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global In-Vitro Toxicology Assays Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global In-Vitro Toxicology Assays Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global In-Vitro Toxicology Assays Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ajinomoto Health & Nutrition North America, Inc., AngioDynamics, Inc., B. Braun Medical Inc., B. Braun Melsungen AG, Baxter International Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this In-Vitro Toxicology Assays market report include:
- Abcam plc
- Agilent Technologies Inc.
- Bio-Rad Laboratories Inc.
- BioIVT
- BioVision Inc.
- Charles River Laboratories
- Creative Bioarray
- Cyprotex (Evotec Group)
- Eurofins Scientific
- GE Healthcare Life Sciences
- Gentronix Ltd
- HemoGenix Inc.
- Merck KGaA (MilliporeSigma)
- PerkinElmer Inc.
- Promega Corporation
- QIAGEN N.V.
- Sekisui XenoTech LLC
- SGS SA
- Thermo Fisher Scientific
- Toxikon Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam plc
- Agilent Technologies Inc.
- Bio-Rad Laboratories Inc.
- BioIVT
- BioVision Inc.
- Charles River Laboratories
- Creative Bioarray
- Cyprotex (Evotec Group)
- Eurofins Scientific
- GE Healthcare Life Sciences
- Gentronix Ltd
- HemoGenix Inc.
- Merck KGaA (MilliporeSigma)
- PerkinElmer Inc.
- Promega Corporation
- QIAGEN N.V.
- Sekisui XenoTech LLC
- SGS SA
- Thermo Fisher Scientific
- Toxikon Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 481 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
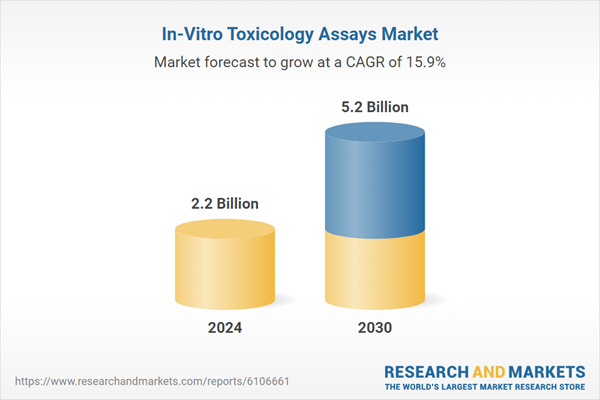
| Estimated Market Value ( USD | $ 2.2 Billion |
| Forecasted Market Value ( USD | $ 5.2 Billion |
| Compound Annual Growth Rate | 15.9% |
| Regions Covered | Global |