Global Janus Kinase (JAK) Inhibitors Market - Key Trends & Drivers Summarized
Why Are Janus Kinase Inhibitors Revolutionizing the Management of Inflammatory and Autoimmune Diseases?
Janus kinase (JAK) inhibitors have rapidly emerged as a transformative therapeutic option in the management of a broad range of chronic inflammatory and autoimmune conditions due to their targeted mechanism of action, rapid symptom control, and oral availability. These small-molecule drugs interrupt intracellular signaling pathways mediated by cytokines, which are central to the pathogenesis of disorders like rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, atopic dermatitis, and ankylosing spondylitis. By modulating the JAK-STAT signaling cascade, JAK inhibitors can downregulate immune overactivity without the broad immunosuppressive effects associated with corticosteroids or traditional disease-modifying antirheumatic drugs (DMARDs). Their oral administration also offers an appealing alternative to injectable biologics, improving adherence and convenience for patients. Clinicians increasingly view JAK inhibitors as a valuable option for patients with refractory disease or those who fail to respond adequately to existing biologics. Additionally, many of these therapies demonstrate rapid onset of action, providing symptom relief within days or weeks, which is crucial for improving quality of life and reducing disability. Regulatory approvals for newer indications such as alopecia areata and vitiligo further underscore the expanding clinical footprint of this drug class. With chronic autoimmune conditions rising globally due to lifestyle changes, environmental factors, and improved diagnosis rates, the role of JAK inhibitors is becoming increasingly central in both frontline and second-line treatment strategies.How Is Innovation in Molecular Targeting and Formulation Driving the Evolution of JAK Inhibitor Therapies?
The scientific landscape surrounding JAK inhibitors is evolving rapidly as researchers refine selectivity, enhance safety profiles, and expand the scope of therapeutic indications. Initial generations of JAK inhibitors were pan-inhibitors, targeting multiple JAK isoforms simultaneously, which raised concerns about off-target effects and infections. However, the latest wave of development focuses on isoform-specific inhibitors that preferentially target JAK1, JAK2, or TYK2, aiming to reduce adverse events such as thromboembolic complications and lipid abnormalities. Pharmaceutical companies are employing advanced molecular modeling and structure-based drug design to create compounds with greater binding precision and metabolic stability. Extended-release formulations and dual-action molecules are being explored to improve pharmacokinetics and allow once-daily or even weekly dosing regimens. Combination therapies that pair JAK inhibitors with biologics or conventional DMARDs are under clinical evaluation to improve efficacy in complex cases. Additionally, topical formulations are being developed for dermatological conditions, allowing localized treatment with reduced systemic exposure. Companion diagnostics and pharmacogenomic tools are also being integrated into clinical workflows to better match patients with the most appropriate JAK inhibitor based on genetic profiles and disease biomarkers. The robust clinical pipeline includes candidates for hard-to-treat conditions such as lupus, multiple sclerosis, graft-versus-host disease, and type 1 diabetes. These innovations are not only improving therapeutic outcomes but also expanding the potential patient base, reinforcing JAK inhibitors' position as a cornerstone of precision immunotherapy.How Do Regulatory Trends and Safety Concerns Influence the Commercial Outlook for JAK Inhibitors?
Regulatory agencies play a critical role in shaping the commercial and clinical trajectory of JAK inhibitors, particularly as safety concerns emerge in long-term post-marketing surveillance. The US FDA, EMA, and other health authorities have implemented heightened scrutiny following signals of increased cardiovascular events, malignancies, and serious infections in patients treated with certain JAK inhibitors. As a result, boxed warnings and restricted indications have been added to drug labels, and risk evaluation and mitigation strategies (REMS) have been introduced to guide prescribing practices. These regulatory responses have prompted pharmaceutical companies to invest heavily in real-world evidence studies and post-approval registries to monitor patient safety across broader populations. In parallel, payers are becoming more selective in their reimbursement criteria, requiring evidence of superior benefit-risk profiles before approving coverage, especially when lower-cost biologics or biosimilars are available. Despite these hurdles, demand remains strong due to the therapeutic value of JAK inhibitors in patient populations that are non-responsive or intolerant to other therapies. Market access strategies now often include education programs for healthcare professionals, digital adherence tools, and partnerships with advocacy groups to support informed decision-making. Additionally, regional regulatory divergence is influencing global commercialization efforts, with faster approvals and broader indications granted in some emerging markets where access to biologics is limited. While the safety narrative presents a challenge, it is also catalyzing industry innovation to produce safer, more selective compounds and to differentiate JAK inhibitors from both traditional immunosuppressants and newer biologics.What Factors Are Driving the Continued Growth of the Global JAK Inhibitors Market?
The growth in the Janus kinase (JAK) inhibitors market is driven by several interrelated factors spanning clinical, technological, economic, and demographic dimensions. Rising prevalence of autoimmune and chronic inflammatory conditions, particularly in aging populations and industrialized nations, is expanding the eligible patient pool for JAK-targeted therapies. Patient preference for oral medications over injectables is pushing physicians to prescribe JAK inhibitors more frequently, especially for individuals requiring long-term treatment. Pharmaceutical innovation is continuously enhancing drug safety, efficacy, and tolerability, resulting in a steady pipeline of next-generation JAK inhibitors with differentiated profiles. Expanding label approvals into dermatology, gastroenterology, and rare autoimmune diseases are increasing product utility across multiple specialties. Competitive dynamics are also contributing to market expansion, as biosimilars and generic alternatives place downward pressure on pricing, making therapies more accessible in middle-income regions. Increased physician awareness, better diagnostic tools, and improved disease classification are enabling earlier intervention with targeted treatments like JAK inhibitors. Health systems under pressure to deliver outcomes at lower costs are recognizing the value of these drugs in reducing hospitalization, surgeries, and long-term complications. Payer models are evolving to reward real-world effectiveness, which favors fast-acting therapies with proven patient-reported outcomes. Global investment in immunology research, combined with the decentralization of clinical trials to reach more diverse populations, is accelerating innovation and market reach. These combined forces are ensuring that JAK inhibitors will remain a high-growth segment of the pharmaceutical industry, meeting the urgent needs of patients while driving revenue opportunities for drug developers worldwide.Report Scope
The report analyzes the Janus Kinase (JAK) Inhibitors market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Autoimmune Disorders Application, Oncology Application).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Autoimmune Disorders Application segment, which is expected to reach US$22.9 Billion by 2030 with a CAGR of a 20.7%. The Oncology Application segment is also set to grow at 14.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.2 Billion in 2024, and China, forecasted to grow at an impressive 25.6% CAGR to reach $7.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Janus Kinase (JAK) Inhibitors Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Janus Kinase (JAK) Inhibitors Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Janus Kinase (JAK) Inhibitors Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Artocarpus Foods Pvt. Ltd., Big Mountain Foods, BoTree Farm Products, Delecta Fruit SA, Fairtrasa International AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Janus Kinase (JAK) Inhibitors market report include:
- AbbVie Inc.
- Aclaris Therapeutics, Inc.
- AstraZeneca plc
- Bristol-Myers Squibb Company
- Celon Pharma S.A.
- Confluence Life Sciences, Inc.
- CTI BioPharma Corp.
- Eli Lilly and Company
- Gilead Sciences, Inc.
- Incyte Corporation
- Italfarmaco S.p.A.
- Jiangsu Hengrui Pharmaceuticals Co.
- Pfizer Inc.
- Prometheus Biosciences, Inc.
- Rigel Pharmaceuticals, Inc.
- Sierra Oncology, Inc.
- Sino Biopharmaceutical Limited
- SOTIO Biotech
- Sun Pharmaceutical Industries Ltd.
- Tiziana Life Sciences Ltd
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Aclaris Therapeutics, Inc.
- AstraZeneca plc
- Bristol-Myers Squibb Company
- Celon Pharma S.A.
- Confluence Life Sciences, Inc.
- CTI BioPharma Corp.
- Eli Lilly and Company
- Gilead Sciences, Inc.
- Incyte Corporation
- Italfarmaco S.p.A.
- Jiangsu Hengrui Pharmaceuticals Co.
- Pfizer Inc.
- Prometheus Biosciences, Inc.
- Rigel Pharmaceuticals, Inc.
- Sierra Oncology, Inc.
- Sino Biopharmaceutical Limited
- SOTIO Biotech
- Sun Pharmaceutical Industries Ltd.
- Tiziana Life Sciences Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 179 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
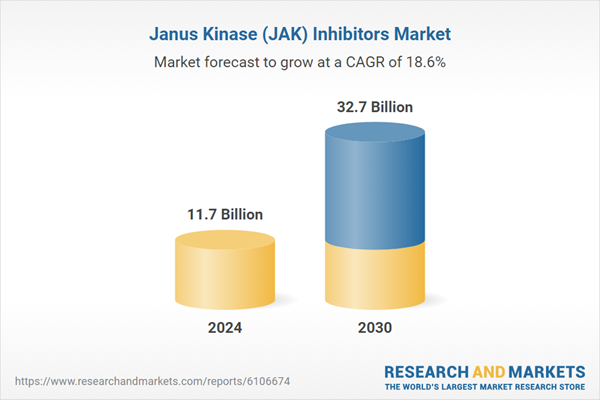
| Estimated Market Value ( USD | $ 11.7 Billion |
| Forecasted Market Value ( USD | $ 32.7 Billion |
| Compound Annual Growth Rate | 18.6% |
| Regions Covered | Global |