Global Mammalian Cell Banking Market - Key Trends & Drivers Summarized
Why Is Mammalian Cell Banking a Cornerstone in Biopharmaceutical Development and Manufacturing?
Mammalian cell banking has become an essential pillar in the development and commercial production of biopharmaceuticals due to its role in preserving and reproducing genetically consistent and contamination-free cell lines. These cell banks serve as the foundational source for producing complex biological products such as monoclonal antibodies, recombinant proteins, vaccines, and gene therapies. Unlike microbial systems, mammalian cells are uniquely capable of performing intricate post-translational modifications, which are critical for the therapeutic efficacy and stability of many biologics. As the global demand for biologics continues to rise, the importance of well-characterized and stable cell lines that ensure product consistency and safety has grown exponentially. Master cell banks (MCBs) and working cell banks (WCBs) are meticulously created under Good Manufacturing Practices (GMP) to guarantee reproducibility in large-scale production while complying with stringent regulatory requirements. Proper storage, documentation, and traceability are critical components of cell banking operations, as any variation can lead to costly delays, failed batches, or compromised patient safety. As biopharma pipelines expand with more targeted and personalized therapies, the role of mammalian cell banking is also broadening to include support for autologous and allogeneic cell therapies. The strategic value of establishing robust cell banks lies in reducing long-term development risks, streamlining scale-up, and enabling faster response to regulatory audits and product recalls. These capabilities highlight why mammalian cell banking is indispensable for both early-stage research and full-scale commercial production across the biologics landscape.How Are Technological Innovations Enhancing the Efficiency and Reliability of Cell Banking Processes?
Advancements in bioprocessing technologies, automation, and analytics are significantly enhancing the precision, scalability, and reliability of mammalian cell banking operations. Innovations in cryopreservation techniques, such as controlled-rate freezing and cryoprotectant optimization, are improving cell viability and genetic stability post-thaw, which is critical for preserving the integrity of both master and working cell banks over time. Automation in cell culture, harvesting, and aliquoting processes reduces the risk of contamination and human error while boosting operational throughput and consistency. Modern biobanking facilities are increasingly utilizing robotics and closed-system technologies to support sterile processing and minimize manual interventions. In addition, advanced analytics such as next-generation sequencing and real-time PCR are being applied to thoroughly characterize cell lines, ensuring they are free from adventitious agents, mycoplasma, and genetic drift. Artificial intelligence and machine learning are beginning to play a role in predictive maintenance and quality forecasting for biobanks, allowing facilities to monitor trends and preempt equipment or viability issues before they compromise product integrity. Cloud-based data management platforms are being adopted to centralize records, enable real-time collaboration across sites, and support regulatory audits with full traceability. These technologies not only streamline operations but also enhance compliance with evolving international standards from regulatory bodies such as the FDA, EMA, and ICH. As a result, companies can significantly reduce time to market while maintaining high standards of quality and reproducibility, thereby reinforcing the strategic importance of investing in technologically advanced mammalian cell banking systems.What Industry Trends Are Shaping the Demand for Mammalian Cell Banking Services Globally?
A variety of industry trends are contributing to increased demand for mammalian cell banking services across the globe, especially as biologics and cell-based therapies become central to modern medicine. The surge in demand for monoclonal antibodies, biosimilars, and personalized medicines is prompting both large biopharmaceutical firms and contract development and manufacturing organizations (CDMOs) to invest in scalable and reliable cell banking infrastructure. The rapid expansion of cell and gene therapy pipelines is also driving a need for customized cell banking solutions that can support autologous and allogeneic workflows under rigorous quality controls. Additionally, as biopharmaceutical innovation expands into rare diseases and orphan drugs, companies are increasingly banking smaller, highly specialized cell lines that must meet strict regulatory and safety benchmarks. The globalization of biologics manufacturing is leading to the establishment of regional cell banking facilities to support decentralized production models, improve supply chain resilience, and meet local regulatory requirements. Meanwhile, the rise of start-ups and academic research institutions in the biotech space is fueling the growth of third-party service providers offering turnkey cell banking solutions. These services include not only cryopreservation and storage but also cell line characterization, documentation, and regulatory support. The shift toward outsourcing and partnership models is enabling smaller players to access high-quality infrastructure without large capital investments. As the biopharma industry becomes more data-driven, demand is growing for integrated solutions that combine biological storage with digital monitoring, compliance tracking, and analytics. Together, these trends are expanding the scope and scale of the global mammalian cell banking market.What Factors Are Driving Growth in the Global Mammalian Cell Banking Market?
The growth in the global mammalian cell banking market is driven by several critical factors related to biopharmaceutical expansion, regulatory compliance, and technological progress. One of the primary drivers is the rising number of biopharmaceutical products entering clinical development and commercialization, especially complex biologics that require mammalian systems for expression. The increasing reliance on Chinese Hamster Ovary (CHO) cells and other mammalian hosts for producing therapeutic proteins is directly tied to the need for robust and compliant cell banking systems. Regulatory expectations around biosafety, traceability, and product consistency are becoming more stringent, compelling biopharma companies to invest in high-quality cell banking facilities and services. Additionally, the growth of personalized medicine, particularly in oncology and regenerative medicine, is creating demand for patient-specific cell lines that must be stored and managed under carefully controlled conditions. Expansion of CDMOs offering specialized cell banking services is making it easier for companies to scale operations and enter global markets more quickly. Furthermore, increased government funding and public-private partnerships in biomanufacturing are supporting infrastructure development and encouraging adoption of GMP-grade biobanking practices. The rise in clinical trials involving stem cell therapies, immunotherapies, and gene editing platforms such as CRISPR is also contributing to the need for secure and scalable mammalian cell storage. Technological advances in cryopreservation, digital tracking, and cell line authentication are enhancing the value proposition for both in-house and outsourced cell banking solutions. Collectively, these factors are positioning mammalian cell banking as a foundational element in the future of biologics development, manufacturing, and commercialization.Report Scope
The report analyzes the Mammalian Cell Banking market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (TEP Technology, IEP Technology, SEP Technology); Application (MCB Application, WCB Application, EoP Application, R&D CB Application); End-Use (Academic & Research Institute End-Use, Biopharma Companies End-Use, CROs End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the TEP Technology segment, which is expected to reach US$621.8 Million by 2030 with a CAGR of a 13.2%. The IEP Technology segment is also set to grow at 15.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $131.5 Million in 2024, and China, forecasted to grow at an impressive 18.5% CAGR to reach $224.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Mammalian Cell Banking Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Mammalian Cell Banking Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Mammalian Cell Banking Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as American Crew (Revlon), Beiersdorf AG, Bulldog Skincare for Men, Colgate-Palmolive Company, Coty Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Mammalian Cell Banking market report include:
- 3P Biopharmaceuticals
- Advanced Therapies
- Becton, Dickinson and Company (BD)
- Charles River Laboratories
- Corning Incorporated
- Cryo-Cell International
- Danaher Corporation
- Eppendorf AG
- Eurofins DiscoverX / SwissBio
- Fujifilm Irvine Scientific
- GE Healthcare Life Sciences (Cytiva)
- InvivoGen
- Lonza Group Ltd
- Merck KGaA (MilliporeSigma)
- Sartorius AG
- Sigma-Aldrich Corporation
- SCTbio
- Takara Bio Inc.
- Thermo Fisher Scientific
- WuXi AppTec / WuXi Biologics
- Cedarlane Laboratories
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3P Biopharmaceuticals
- Advanced Therapies
- Becton, Dickinson and Company (BD)
- Charles River Laboratories
- Corning Incorporated
- Cryo-Cell International
- Danaher Corporation
- Eppendorf AG
- Eurofins DiscoverX / SwissBio
- Fujifilm Irvine Scientific
- GE Healthcare Life Sciences (Cytiva)
- InvivoGen
- Lonza Group Ltd
- Merck KGaA (MilliporeSigma)
- Sartorius AG
- Sigma-Aldrich Corporation
- SCTbio
- Takara Bio Inc.
- Thermo Fisher Scientific
- WuXi AppTec / WuXi Biologics
- Cedarlane Laboratories
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 377 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
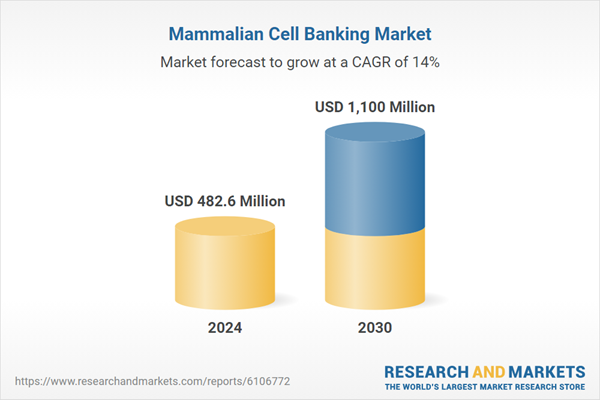
| Estimated Market Value ( USD | $ 482.6 Million |
| Forecasted Market Value ( USD | $ 1100 Million |
| Compound Annual Growth Rate | 14.0% |
| Regions Covered | Global |