Global Mono Vaccines Market - Key Trends & Drivers Summarized
Why Are Mono Vaccines Still Relevant in Modern Immunization Strategies?
Mono vaccines, which are designed to immunize against a single pathogen, remain an essential component of global vaccination programs. These vaccines are widely used in both routine immunization schedules and targeted public health campaigns. Their relevance is especially clear in early childhood immunization, travel medicine, and outbreak control where specific disease protection is required. Common mono vaccines include those for measles, hepatitis B, influenza, tetanus, and rabies.Mono vaccines offer precise protection without exposing the immune system to multiple antigens simultaneously. This is particularly important in certain clinical situations, such as post-exposure prophylaxis or when tailoring immunization based on patient history, allergies, or contraindications. Health authorities continue to rely on mono vaccines for phased immunization schedules, booster doses, and targeted interventions in disease elimination efforts.
How Is Technology Influencing Mono Vaccine Development and Production?
Technological advancements in vaccine development have improved the safety, stability, and effectiveness of mono vaccines. Modern production techniques include recombinant DNA technology, virus-like particle platforms, and mRNA-based development, all of which contribute to improved immune response and reduced side effects. Innovations in adjuvants and delivery systems are also enhancing antigen presentation and long-term immunity.Cold chain optimization and lyophilized formulations have increased the logistical viability of mono vaccines in low-resource settings. Standardization in dosage and needle-free delivery options, such as microneedle patches and oral vaccines, are also being developed to improve compliance and ease of administration. Increasing use of cell-culture production and automation in biomanufacturing is helping scale up production efficiently and cost-effectively.
Where Is Demand Rising and Who Are the Primary End Users?
Demand for mono vaccines is increasing in pediatric healthcare, travel medicine, public health outreach, and veterinary applications. National immunization programs across both developed and developing countries include mono vaccines for diseases that still pose public health threats. In areas where outbreaks occur, mono vaccines are rapidly deployed to control disease spread and protect vulnerable populations.Hospitals, clinics, and government health departments are the primary end users, alongside non-governmental organizations running disease control campaigns. Travelers and healthcare workers often require mono vaccines specific to regional disease risks. In veterinary medicine, mono vaccines are used to prevent individual diseases in livestock and companion animals, supporting both animal welfare and food safety.
What Factors Are Driving Growth In The Mono Vaccines Market?
Growth in the mono vaccines market is driven by several factors including persistent disease burdens, regulatory focus on targeted immunization, and the need for flexible vaccination options. Outbreaks of diseases such as measles, hepatitis, and rabies continue to sustain demand for rapid, single-antigen immunization. Rising awareness about disease-specific risks and expansion of adult immunization programs are also contributing to growth.Technological progress in vaccine design, more efficient production methods, and public-private collaborations are supporting broader access to affordable and effective mono vaccines. Programs focused on eliminating specific infectious diseases also depend heavily on mono vaccine deployment. As healthcare systems emphasize precision immunization and post-exposure management, mono vaccines are expected to maintain a strong position within global vaccine portfolios.
Report Scope
The report analyzes the Mono Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Meningococcal Vaccine, Japanese Encephalitis Vaccine, Hepatitis Vaccine, Yellow Fever Vaccine, Other Types).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Meningococcal Vaccine segment, which is expected to reach US$5.2 Billion by 2030 with a CAGR of a 3.3%. The Japanese Encephalitis Vaccine segment is also set to grow at 3.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.8 Billion in 2024, and China, forecasted to grow at an impressive 5.8% CAGR to reach $2.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Mono Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Mono Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Mono Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACE Money Transfer, Azimo, BFC Group, CurrencyFair, Euronet Worldwide (Ria) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Mono Vaccines market report include:
- AstraZeneca Plc
- Bavarian Nordic A/S
- Biological E Limited
- CanSino Biologics Inc.
- CSL Seqirus (CSL Limited)
- Daiichi Sankyo Co., Ltd.
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc (GSK)
- Merck & Co., Inc.
- Moderna, Inc.
- Novavax, Inc.
- Panacea Biotec Ltd
- Pfizer Inc.
- Sanofi Pasteur (Sanofi S.A.)
- Serum Institute of India Pvt. Ltd.
- Sinovac Biotech Ltd.
- Takeda Pharmaceutical Co., Ltd.
- Valneva SE
- VBI Vaccines Inc.
- Zydus Lifesciences Ltd. (Zydus Cadila)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AstraZeneca Plc
- Bavarian Nordic A/S
- Biological E Limited
- CanSino Biologics Inc.
- CSL Seqirus (CSL Limited)
- Daiichi Sankyo Co., Ltd.
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc (GSK)
- Merck & Co., Inc.
- Moderna, Inc.
- Novavax, Inc.
- Panacea Biotec Ltd
- Pfizer Inc.
- Sanofi Pasteur (Sanofi S.A.)
- Serum Institute of India Pvt. Ltd.
- Sinovac Biotech Ltd.
- Takeda Pharmaceutical Co., Ltd.
- Valneva SE
- VBI Vaccines Inc.
- Zydus Lifesciences Ltd. (Zydus Cadila)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
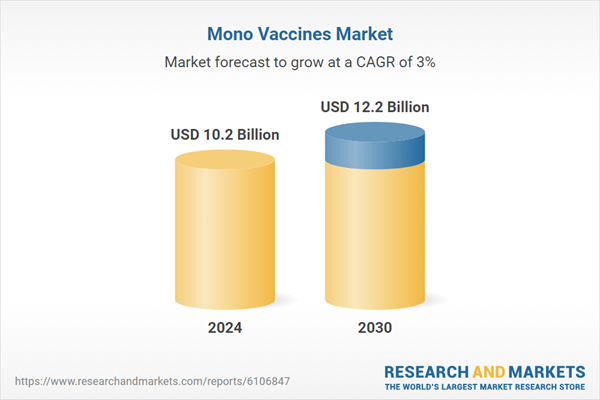
| Estimated Market Value ( USD | $ 10.2 Billion |
| Forecasted Market Value ( USD | $ 12.2 Billion |
| Compound Annual Growth Rate | 3.0% |
| Regions Covered | Global |