Global Tertiary Dilation Balloon Catheters Market - Key Trends & Drivers Summarized
Are Tertiary Dilation Balloon Catheters Shaping the Next Frontier of Minimally Invasive Interventions?
Tertiary dilation balloon catheters represent a highly specialized segment within interventional medical devices, particularly suited for complex endoscopic and cardiovascular procedures. Unlike primary or secondary balloons that focus on initial vessel or tract opening, tertiary balloons are used for fine-tuning dilation, addressing resistant strictures, or navigating anatomically complex areas. Their design emphasizes precision, repeatability, and control, making them essential in high-stakes interventions involving the esophagus, biliary tract, or peripheral arteries. As the demand for minimally invasive solutions grows across global healthcare systems, these advanced catheters are gaining recognition for their role in reducing procedural risks and improving patient outcomes.A major driver behind their increased use is the global shift toward non-surgical treatment pathways for chronic and progressive conditions such as atherosclerosis, gastrointestinal strictures, and urological obstructions. Tertiary balloon catheters are especially useful in scenarios requiring incremental and staged dilation, where a gentle, controlled approach is needed to prevent tissue trauma or perforation. Their growing application in combination therapies-such as with drug-coated balloons or stents-is also elevating their clinical utility. As a result, they are no longer niche tools reserved for complex cases, but increasingly included in mainstream treatment protocols, particularly in tertiary and quaternary care centers worldwide.
How Are Design Enhancements and Clinical Versatility Advancing Tertiary Balloon Catheter Adoption?
Technological innovation is playing a central role in the rising adoption of tertiary dilation balloon catheters. Advancements in balloon material-such as high-compliance polymers and pressure-resistant compounds-have enabled catheters to maintain integrity at variable inflation levels, ensuring consistent performance even in tortuous anatomy. These newer designs offer enhanced pushability, trackability, and kink resistance, attributes that are vital during difficult navigation through stenotic pathways. Multi-stage balloons, dual-lumen structures, and rapid exchange systems are further improving procedural speed and precision, critical for reducing catheterization time and lowering patient risk.Beyond cardiovascular use, tertiary balloon catheters are increasingly used in gastroenterology and urology for strictures in the esophagus, urethra, and bile ducts. Their ability to provide radial force in a controlled manner makes them indispensable in treating complex or recurrent narrowing. Furthermore, the inclusion of radiopaque markers and compatibility with imaging modalities like fluoroscopy and endoscopic ultrasound (EUS) allows for real-time tracking and positional accuracy. In pediatric applications, specially calibrated tertiary balloons enable gradual dilation in congenital anomalies without the complications associated with aggressive expansion. This broad clinical utility across age groups and specialties has solidified their position as essential instruments in modern interventional toolkits.
What Role Is Digitally-Enhanced Procedural Control Playing in Market Evolution?
In parallel with material advancements, the digital transformation of interventional procedures is adding a new dimension to tertiary balloon catheter performance. The integration of digital inflation devices, pressure monitoring systems, and smart feedback mechanisms enables real-time data capture on balloon behavior, pressure gradients, and dilation success rates. This information is crucial for fine-tuning procedural strategy, especially in complex vascular and non-vascular interventions. The growing use of AI-assisted imaging software is also enhancing catheter placement accuracy, ensuring optimal balloon deployment in targeted zones without overshoot or under-dilation.Additionally, manufacturers are investing in catheter systems that are compatible with robotic-assisted platforms and hybrid OR suites. These systems allow for highly controlled, remote-guided dilation procedures, which are particularly beneficial in high-risk or hard-to-access anatomical regions. Modular catheter systems with interchangeable balloon sizes and lengths are being developed to cater to procedure-specific requirements, reducing inventory burden while increasing flexibility. In research settings, smart materials capable of responding to biological cues are being explored to create responsive balloon systems that adapt inflation parameters automatically. While still in developmental stages, such innovations hint at a future where tertiary dilation procedures are not just safer and more efficient-but also semi-autonomous and predictive in their delivery.
What Is Fueling the Growth of the Tertiary Dilation Balloon Catheters Market?
The growth in the tertiary dilation balloon catheters market is driven by several factors closely tied to procedural advancements, application diversity, and digital health integration. First, the increasing global preference for minimally invasive techniques is significantly raising demand for precise, low-trauma tools like tertiary balloons, particularly in cardiovascular and endoscopic surgeries. Second, the rising incidence of chronic diseases such as peripheral artery disease, esophageal cancer, and biliary obstructions is expanding the number of cases requiring stepwise or staged dilation interventions, where tertiary balloons are most effective.Third, innovation in catheter engineering-such as improved balloon compliance, variable diameter systems, and enhanced radiopacity-has improved performance in anatomically challenging and high-risk procedures. Fourth, the development of integrated systems featuring real-time pressure feedback, digital inflation monitoring, and imaging-guided deployment has brought procedural control to new levels, increasing surgeon confidence and procedural efficacy. Fifth, the expansion of healthcare infrastructure in emerging economies is enabling more complex procedures to be performed in non-urban and mid-tier hospitals, increasing the accessibility and usage of such high-precision devices. Lastly, regulatory approvals and reimbursement expansions for advanced dilation procedures are accelerating clinical adoption in both public and private sectors. Together, these drivers point to a robust, high-value market trajectory defined by innovation, precision, and broader clinical applicability.
Scope Of Study:
The report analyzes the Tertiary Dilation Balloon Catheters market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Type (Silicone Balloon Catheters, Polyethylene Balloon Catheters); Application (Heart Disease Application, Vascular Disease Application, Digestive Tract Disease Application, Urinary System Disease Application, Gynecological Disease Application); End-Use (Hospitals End-Use, Ambulatory Surgery Centers End-Use, Other End-Uses)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Silicone Balloon Catheters segment, which is expected to reach US$592.5 Million by 2030 with a CAGR of a 3.1%. The Polyethylene Balloon Catheters segment is also set to grow at 5.0% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $200.1 Million in 2024, and China, forecasted to grow at an impressive 6.8% CAGR to reach $182.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Tertiary Dilation Balloon Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Tertiary Dilation Balloon Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Tertiary Dilation Balloon Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories (OrbusNeich), AndraTec GmbH, B. Braun Melsungen AG, Becton Dickinson (BD), Biotronik, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Tertiary Dilation Balloon Catheters market report include:
- Abbott Laboratories (OrbusNeich)
- AndraTec GmbH
- B. Braun Melsungen AG
- Becton Dickinson (BD)
- Biotronik, Inc.
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cook Medical Inc.
- Duke Empirical
- LeMaitre Vascular
- Medtronic plc
- Natec Medical
- OrbusNeich Medical Group
- Pergan GmbH
- Polytech Health & Aesthetics (PVA)
- Surmodics, Inc.
- Teleflex Incorporated
- Terumo Corporation
- United Initiators GmbH
- Vascular Solutions, Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories (OrbusNeich)
- AndraTec GmbH
- B. Braun Melsungen AG
- Becton Dickinson (BD)
- Biotronik, Inc.
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cook Medical Inc.
- Duke Empirical
- LeMaitre Vascular
- Medtronic plc
- Natec Medical
- OrbusNeich Medical Group
- Pergan GmbH
- Polytech Health & Aesthetics (PVA)
- Surmodics, Inc.
- Teleflex Incorporated
- Terumo Corporation
- United Initiators GmbH
- Vascular Solutions, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 368 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
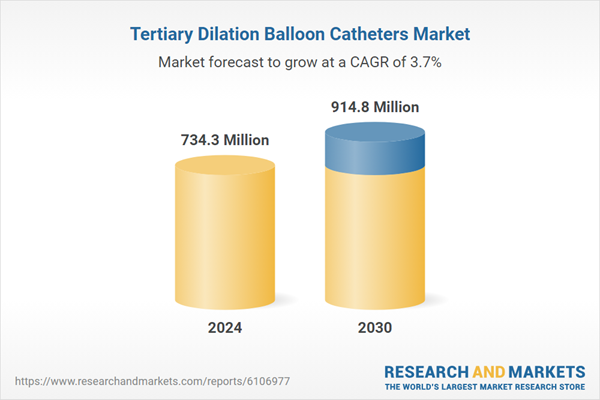
| Estimated Market Value in 2024 | 734.3 Million |
| Forecasted Market Value by 2030 | 914.8 Million |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |