Global Urea Cycle Disorder Treatment Market - Key Trends & Drivers Summarized
Why Is Effective Treatment for Urea Cycle Disorder Becoming Increasingly Crucial?
Urea cycle disorder (UCD) is a rare but serious group of inherited metabolic conditions that impair the body's ability to eliminate ammonia, a toxic byproduct of protein metabolism. As awareness of genetic diseases grows and neonatal screening becomes more widespread, earlier diagnosis of UCD is driving greater attention toward timely and effective treatment options. Although the disorder is rare, its consequences can be life-threatening if left unmanaged, leading to irreversible brain damage, coma, or death, particularly in newborns and young children. This reality makes the development and availability of safe and efficient treatment protocols an urgent public health concern. UCD may present suddenly in infants or develop more gradually in adolescents and adults, which complicates diagnosis and often leads to delayed intervention. With improved genetic testing and metabolic screening methods, more cases are being identified early, resulting in increased demand for therapeutic interventions that can control ammonia levels and prevent acute hyperammonemic crises. The chronic nature of UCD also means that patients require lifelong management strategies, which often involve dietary control, ammonia scavenger medications, amino acid supplementation, and in severe cases, liver transplantation. The expanding diagnostic landscape, coupled with the growing number of identified cases through family screening and newborn panels, highlights why UCD treatment is becoming a more prominent focus within the rare disease and metabolic disorder space.How Are Treatment Innovations and Drug Developments Transforming UCD Management?
Treatment options for urea cycle disorder have advanced significantly in recent years, thanks to targeted research efforts, orphan drug designations, and deeper understanding of the disorder's biochemical pathways. Traditional management strategies primarily involve protein-restricted diets to reduce ammonia production, supplemented with nitrogen-scavenging agents like sodium benzoate and sodium phenylbutyrate, which help remove excess nitrogen through alternative pathways. More recently, novel formulations such as glycerol phenylbutyrate have emerged, offering improved pharmacokinetics, better patient compliance, and reduced dosing frequency. These newer drugs are particularly valuable in pediatric care, where palatability and ease of administration are critical to long-term success. Additionally, therapeutic developments are focusing on restoring urea cycle function or compensating for the missing enzyme activity at a cellular level. Gene therapy and mRNA-based treatments are being actively explored, with preclinical and early-phase clinical trials showing promise for long-term correction of enzyme deficiencies. Enzyme replacement therapy is another avenue under investigation, aimed at reducing dependence on lifelong medication and strict dietary controls. Advances in metabolic monitoring tools are also improving treatment personalization, allowing clinicians to adjust therapeutic regimens based on real-time data from ammonia levels, amino acid concentrations, and patient metabolic responses. These innovations are transforming UCD from a condition that once had limited treatment options into a disease area where targeted and potentially curative therapies are on the horizon.What Role Do Healthcare Systems, Patient Advocacy, and Access to Diagnosis Play in Market Dynamics?
The management and treatment of urea cycle disorder are deeply influenced by the structure and responsiveness of healthcare systems, availability of diagnostic tools, and advocacy efforts from patient communities. In regions with well-developed neonatal screening programs, such as the United States and parts of Europe, early diagnosis and intervention significantly improve clinical outcomes, reduce the risk of cognitive impairment, and increase the lifespan and quality of life for patients. Access to specialized metabolic centers and trained healthcare providers also plays a crucial role in ensuring optimal treatment, as UCD requires multidisciplinary care involving dietitians, geneticists, neurologists, and metabolic specialists. In low- and middle-income countries, however, limited awareness and inadequate infrastructure often result in underdiagnosis or misdiagnosis, with many cases going untreated until symptoms become severe. Patient advocacy groups are stepping in to bridge these gaps by raising awareness, supporting education for healthcare professionals, and lobbying for inclusion of UCD in national newborn screening panels. These organizations also contribute by funding research, facilitating access to clinical trials, and helping patients navigate reimbursement and insurance processes for costly treatments. Access to genetic counseling and family testing further supports disease management by enabling early identification of at-risk individuals. Collectively, the role of healthcare infrastructure and community support is instrumental in shaping how UCD is diagnosed, treated, and managed across different regions and populations.What Are the Key Factors Driving Growth in the Urea Cycle Disorder Treatment Market?
The growth in the urea cycle disorder treatment market is driven by a blend of scientific progress, expanding diagnostic reach, and strategic healthcare initiatives aimed at addressing rare diseases. One of the most critical growth drivers is the rising incidence of diagnosed UCD cases, largely due to improvements in neonatal screening programs and increased genetic awareness. The availability of orphan drug incentives and regulatory fast-track designations is encouraging pharmaceutical companies to invest in research and development, resulting in a growing pipeline of innovative therapies aimed at better efficacy, fewer side effects, and improved patient adherence. Expanding access to metabolic testing and genetic counseling is helping identify both symptomatic and asymptomatic individuals, further increasing treatment uptake. Government and private sector investment in rare disease research is also contributing to the development of novel treatment modalities, including gene therapies and advanced biologics, which have the potential to offer long-term solutions or even curative outcomes. Additionally, the growing role of digital health technologies and remote monitoring tools is enabling more precise and continuous management of ammonia levels, improving clinical decision-making and reducing the need for hospitalizations. As patient awareness continues to grow and global healthcare systems prioritize rare disease coverage, demand for UCD treatments is expected to rise steadily. These interrelated factors are driving a sustained and promising expansion of the global urea cycle disorder treatment market, supported by both clinical necessity and innovation momentum.Scope Of Study:
The report analyzes the Urea Cycle Disorder Treatment market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Therapy (Glycerol Phenylbutyrate Therapy, Sodium Phenylbutyrate Therapy, Amino Acid Supplements Therapy, Sodium Benzoate Therapy, Other Therapies); Administration Route (Oral Administration, Injectable Administration); End-Use (Hospitals End-Use, Specialized Clinics End-Use, Home Care Settings End-Use, Research Institutions End-Use)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Glycerol Phenylbutyrate Therapy segment, which is expected to reach US$565.1 Million by 2030 with a CAGR of a 3.0%. The Sodium Phenylbutyrate Therapy segment is also set to grow at 3.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $353.6 Million in 2024, and China, forecasted to grow at an impressive 4.9% CAGR to reach $289.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Urea Cycle Disorder Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Urea Cycle Disorder Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Urea Cycle Disorder Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acer Therapeutics Inc., Aeglea BioTherapeutics Inc., Arcturus Therapeutics Holdings Inc., Abbott Laboratories Inc., Bausch Health Companies Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Urea Cycle Disorder Treatment market report include:
- Acer Therapeutics Inc.
- Aeglea BioTherapeutics Inc.
- Arcturus Therapeutics Holdings Inc.
- Abbott Laboratories Inc.
- Bausch Health Companies Inc.
- BioMarin Pharmaceutical Inc.
- Censa Pharmaceuticals Inc.
- Danone S.A.
- Eurocept Pharmaceuticals (Lucane Pharma SA)
- Horizon Therapeutics plc
- Mead Johnson & Company, LLC
- Moderna, Inc.
- Orpharma Pty Ltd.
- Precision BioSciences
- Recordati Rare Diseases Inc.
- Relief Therapeutics Holding AG
- Selecta Biosciences Inc.
- Swedish Orphan Biovitrum AB (Sobi)
- Synlogic Inc.
- Ultragenyx Pharmaceutical Inc.
- Xenion Biotech, Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acer Therapeutics Inc.
- Aeglea BioTherapeutics Inc.
- Arcturus Therapeutics Holdings Inc.
- Abbott Laboratories Inc.
- Bausch Health Companies Inc.
- BioMarin Pharmaceutical Inc.
- Censa Pharmaceuticals Inc.
- Danone S.A.
- Eurocept Pharmaceuticals (Lucane Pharma SA)
- Horizon Therapeutics plc
- Mead Johnson & Company, LLC
- Moderna, Inc.
- Orpharma Pty Ltd.
- Precision BioSciences
- Recordati Rare Diseases Inc.
- Relief Therapeutics Holding AG
- Selecta Biosciences Inc.
- Swedish Orphan Biovitrum AB (Sobi)
- Synlogic Inc.
- Ultragenyx Pharmaceutical Inc.
- Xenion Biotech, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 381 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
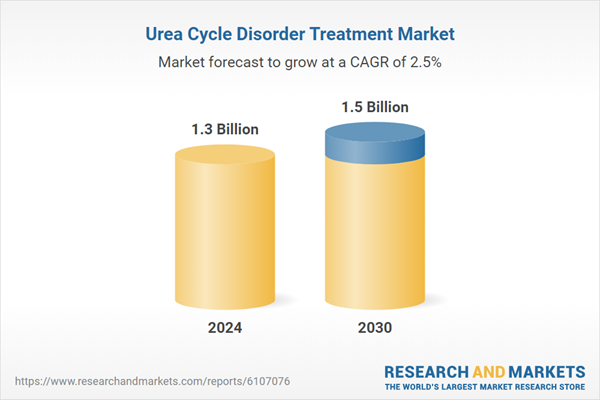
| Estimated Market Value in 2024 | 1.3 Billion |
| Forecasted Market Value by 2030 | 1.5 Billion |
| Compound Annual Growth Rate | 2.5% |
| Regions Covered | Global |