Advancements in digital imaging technologies combined with state-of-the-art surgical instruments are playing a crucial role in accelerating the use of rib repair systems. These innovations provide surgeons with enhanced precision and better visualization during procedures, which leads to improved patient outcomes and shorter recovery times. As a result, rib fracture repair is becoming a preferred solution not only in trauma care but also within orthopedic and thoracic surgery units. The growing confidence in minimally invasive techniques, supported by real-time imaging and improved implant designs, is further driving market expansion.
The anterior plate systems segment was valued at USD 183.2 million in 2024. Their growing popularity comes from their anatomical shaping, which helps minimize tissue irritation and reduce the chance of post-surgical complications. These implants are preferred in patients who require enhanced comfort or for those where aesthetics is a consideration. With surgeons seeking efficient fixation systems that balance functionality and comfort, anterior plates are increasingly selected for both routine and emergency procedures.
The titanium-based systems segment captured a 73.1% share in 2024, largely due to the material’s unmatched strength-to-weight ratio. Titanium offers rigidity without excess hardware bulk, supporting the rib’s natural motion during respiration. Its adaptability to the thoracic structure and proven mechanical consistency help maintain rib alignment effectively throughout the healing process. Titanium’s exceptional biocompatibility and low likelihood of causing immune responses or inflammation make it a safe long-term solution in surgical repairs, fueling its continued dominance in rib fracture repair solutions.
United States Rib Fracture Repair Systems Market was valued at USD 124.8 million in 2024 due to its high rate of chest trauma cases and its advanced healthcare infrastructure. Frequent injuries caused by motor vehicle collisions, aging-related falls, and sports trauma are driving hospital and trauma center demand for rib fixation solutions. With the widespread adoption of high-end surgical technologies, including 3D imaging systems and minimally invasive implants, U.S. institutions are early adopters of cutting-edge rib stabilization technologies. This environment supports the continuous integration of titanium-based, precision-engineered implants throughout surgical centers, contributing to national market expansion.
Key players involved in the market include Able Medical Devices, Acumed, Arthrex, Jeil Medical, Johnson & Johnson, KLS Martin, Medtronic, Neuro France Implants, Orthofix, OsteoMed, Selective Surgical, Smith & Nephew, Stryker, Waston Medical, Zimmer Biomet. Prominent companies in this space are intensifying their focus on developing innovative, low-profile implant designs that meet both surgeon preferences and patient-specific anatomical requirements.
They are also emphasizing R&D to produce biocompatible and durable materials like titanium and hybrid alloys. Strategic partnerships with trauma centers and teaching hospitals help drive early adoption and feedback-driven product refinement. Firms are expanding their global footprint through FDA and CE approvals, enhancing access to minimally invasive implants in new regional markets.
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
Companies Mentioned
- Able Medical Devices
- Acumed
- Arthrex
- Jeil Medical
- Johnson & Johnson
- KLS Martin
- Medtronic
- Neuro France Implants
- Orthofix
- OsteoMed
- Selective Surgical
- Smith & Nephew
- Stryker
- Waston Medical
- Zimmer Biomet
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 130 |
| Published | June 2025 |
| Forecast Period | 2024 - 2034 |
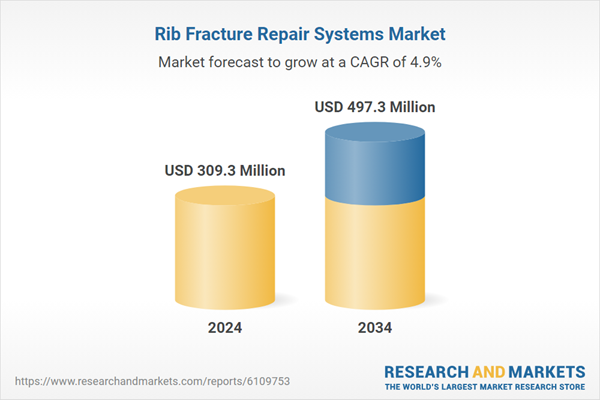
| Estimated Market Value ( USD | $ 309.3 Million |
| Forecasted Market Value ( USD | $ 497.3 Million |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |