Global Recombinant Coagulation Factors Market - Key Trends & Drivers Summarized
How Are Recombinant Technologies Reshaping Coagulation Therapies?
The field of hematology has been significantly transformed by the advancement of recombinant technologies, particularly in the production of coagulation factors used for managing hemophilia and other bleeding disorders. Unlike plasma-derived therapies, recombinant coagulation factors are synthesized using genetically engineered cell lines, eliminating the risks associated with blood-borne pathogens. This technological leap has improved the safety profile of factor VIII and IX treatments, especially among pediatric and immunocompromised populations. Moreover, the increasing adoption of third-generation recombinant factors-which are free from human and animal proteins-has strengthened clinical confidence and regulatory acceptance across developed markets.Further innovation is observed in extended half-life recombinant products, designed to reduce the frequency of infusions and improve patient adherence. These modifications, achieved via PEGylation or fusion protein technology, enhance pharmacokinetics without compromising therapeutic efficacy. As gene therapy research advances, recombinant platforms remain the dominant standard for prophylactic and on-demand treatments, particularly in regions where gene transfer therapies are either cost-prohibitive or still under clinical evaluation. With biosimilar entrants gaining traction in cost-sensitive markets, the landscape is expanding beyond originator products, promoting broader access and supporting health system sustainability.
Which Clinical and Patient-Centric Shifts Are Expanding the Market?
The growing emphasis on prophylactic care and patient quality of life is substantially altering the usage dynamics of recombinant coagulation factors. Clinicians are now favoring long-term preventive regimens that maintain consistent factor levels rather than reactive, event-based infusions. This shift is supported by a growing body of evidence demonstrating that prophylaxis reduces joint damage, hospitalization rates, and total healthcare costs over time. These evolving clinical preferences are stimulating the development of subcutaneous formulations and home-based infusion protocols, which enhance treatment convenience and empower patients to manage their condition autonomously.At the same time, the expansion of neonatal screening programs and early diagnostic frameworks across emerging markets is widening the eligible patient base for recombinant therapies. Rising disease awareness, driven by advocacy groups and government-led initiatives, has led to improved diagnosis of mild and moderate cases that were previously underreported or mismanaged. Pediatric use of recombinant factors is gaining traction, supported by favorable reimbursement schemes and data highlighting reduced inhibitor development compared to plasma-derived alternatives. This trend is particularly pronounced in middle-income countries upgrading their national hemophilia care standards through strategic alliances with multinational biopharma firms.
What Role Do Manufacturing Capabilities and Supply Chain Dynamics Play?
Manufacturing scalability and cold-chain logistics play a central role in shaping access and affordability in the recombinant coagulation factors market. Biomanufacturing of these biologics requires high-yield expression systems, rigorous purification protocols, and validated aseptic conditions. Companies with established production platforms and strong regulatory track records are better positioned to meet rising global demand, particularly in high-prevalence regions such as South Asia and Eastern Europe. Recent capacity expansions, especially in China and India, are geared toward supporting domestic needs while also serving as contract manufacturing hubs for Western firms seeking cost efficiency.Distribution infrastructure and temperature-controlled logistics remain critical for product stability, particularly in tropical and rural areas with unreliable healthcare access. Advances in lyophilized and ready-to-reconstitute formulations are helping mitigate these challenges by improving product shelf-life and field usability. Moreover, collaborations with NGOs and international aid programs-such as the World Federation of Hemophilia’s Humanitarian Aid Program-are extending recombinant product availability in underserved regions. These partnerships, combined with pricing-tier strategies, are lowering market entry barriers and fueling incremental growth beyond traditional high-income markets.
What Is Driving the Sustained Expansion of This Market?
The growth in the recombinant coagulation factors market is driven by several factors, including continuous innovation in recombinant technologies, evolving treatment paradigms favoring prophylactic care, and expanding global access to hematologic therapies. The increasing availability of extended half-life products is a key catalyst, offering improved dosing schedules and patient outcomes. Furthermore, rising investments in hemophilia infrastructure, including treatment centers and registries, are enabling earlier diagnosis and structured long-term care-thereby increasing the addressable treatment population.Biopharmaceutical firms are also accelerating pipeline development for novel recombinant factors that can address rare and inhibitor-resistant bleeding disorders. Parallel to this, favorable regulatory pathways for biosimilars and improved pharmacoeconomic evaluations are expanding market competitiveness, lowering unit costs, and facilitating payer adoption. Technological integration-such as digital infusion tracking, telemedicine for remote monitoring, and AI-based dosing algorithms-is making recombinant therapy more precise and personalized, reinforcing long-term adherence.
Regional growth is further supported by policy incentives, national procurement programs, and value-based reimbursement frameworks that reward outcomes over volume. As global health systems prioritize non-communicable disease management, recombinant coagulation factors are positioned as a cornerstone of modern bleeding disorder care. Collectively, these drivers underpin the robust and sustained trajectory of the global recombinant coagulation factors market.
Scope of the Report
The report analyzes the Recombinant Coagulation Factors market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Recombinant Factor VIII, Recombinant Factor IX, Von Willebrand Factor); Hemophilia Type (Hemophilia A, Hemophilia B); End-Use (Hospitals End-Use, Clinics End-Use, Research Organization End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Recombinant Factor VIII segment, which is expected to reach US$11.7 Billion by 2030 with a CAGR of a 5.6%. The Recombinant Factor IX segment is also set to grow at 3.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.0 Billion in 2024, and China, forecasted to grow at an impressive 8.1% CAGR to reach $3.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Recombinant Coagulation Factors Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Recombinant Coagulation Factors Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Recombinant Coagulation Factors Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Albumedix Ltd., Bayer AG, Baxter International Inc., BDI Pharma, Inc., Biogen Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Recombinant Coagulation Factors market report include:
- Albumedix Ltd.
- Bayer AG
- Baxter International Inc.
- BDI Pharma, Inc.
- Biogen Inc.
- CSL Behring (CSL Limited)
- Emergent BioSolutions Inc.
- Genzyme (Sanofi Genzyme)
- Grifols, S.A.
- Green Cross Corporation
- Kedrion S.p.A.
- LFB S.A.
- Novo Nordisk A/S
- Octapharma AG
- Pfizer Inc.
- Prometic Life Sciences Inc.
- Sanofi S.A.
- Shire (now part of Takeda)
- Swedish Orphan Biovitrum AB (Sobi)
- Takeda Pharmaceutical Company
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Albumedix Ltd.
- Bayer AG
- Baxter International Inc.
- BDI Pharma, Inc.
- Biogen Inc.
- CSL Behring (CSL Limited)
- Emergent BioSolutions Inc.
- Genzyme (Sanofi Genzyme)
- Grifols, S.A.
- Green Cross Corporation
- Kedrion S.p.A.
- LFB S.A.
- Novo Nordisk A/S
- Octapharma AG
- Pfizer Inc.
- Prometic Life Sciences Inc.
- Sanofi S.A.
- Shire (now part of Takeda)
- Swedish Orphan Biovitrum AB (Sobi)
- Takeda Pharmaceutical Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 377 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
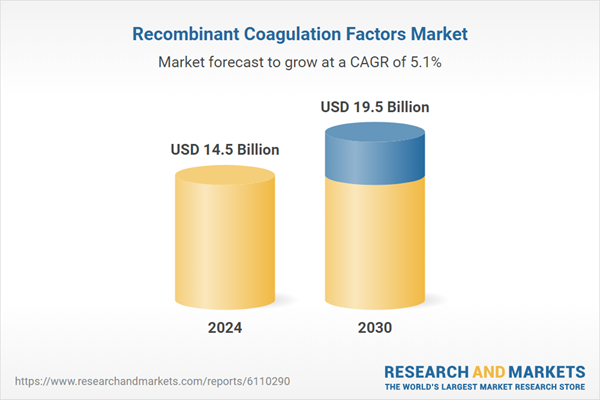
| Estimated Market Value ( USD | $ 14.5 Billion |
| Forecasted Market Value ( USD | $ 19.5 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |