Global Rare Genetic Disorders Treatment Market - Key Trends & Drivers Summarized
Why Are Rare Genetic Disorders Finally Receiving Market Attention?
Rare genetic disorders-once neglected due to small patient populations and limited commercial incentives-are now at the forefront of biopharmaceutical innovation. Characterized by complex pathologies and often lacking effective treatments, these disorders collectively affect over 300 million people worldwide, despite each condition being individually rare. Advances in genomics, CRISPR-Cas9 gene editing, and high-throughput drug screening have created unprecedented opportunities to design targeted therapies, shifting the commercial paradigm in favor of rare disease investment.Pharmaceutical companies are increasingly drawn to this market by orphan drug designations and incentives such as market exclusivity, tax credits, and accelerated regulatory pathways offered by agencies like the U.S. FDA, EMA, and Japan’s PMDA. These incentives have stimulated significant activity in the development of therapies for conditions such as Duchenne muscular dystrophy (DMD), spinal muscular atrophy (SMA), Fabry disease, Gaucher disease, and Rett syndrome. Breakthrough approvals like Zolgensma (gene therapy for SMA) and Evrysdi (SMN2 splicing modulator) exemplify how targeted innovation can address previously untreatable conditions.
How Are Novel Modalities and Precision Approaches Transforming Treatment?
The treatment landscape for rare genetic disorders is being reshaped by gene therapy, antisense oligonucleotides (ASOs), and enzyme replacement therapies (ERTs). Gene therapies-such as AAV-mediated delivery systems-offer one-time, potentially curative interventions for monogenic disorders. These therapies are expanding into broader indications like hemophilia A/B and metabolic syndromes, supported by scalable manufacturing platforms and deeper mechanistic understanding of genetic expression.ASOs, such as those used in treating SMA and Batten disease, provide personalized treatments tailored to individual mutations. These agents modify splicing or gene transcription to restore deficient protein production, offering hope in conditions where traditional small molecules have failed. Meanwhile, ERTs continue to dominate in lysosomal storage disorders, with newer formulations exhibiting improved biodistribution and reduced immunogenicity.
Another notable advancement is the use of patient-derived induced pluripotent stem cells (iPSCs) and organoid models for preclinical drug testing. These enable personalized medicine approaches that align with the mutation-specific etiology of many rare diseases. Clinical trial design is also being revolutionized through basket trials, n-of-1 studies, and natural history data integration-tailored to the small, dispersed nature of rare disease populations.
Which Therapeutic Areas and Stakeholders Are Leading the Push?
Neurological and metabolic genetic disorders represent the highest concentration of innovation and investment. Conditions such as Huntington’s disease, SMA, and Leber congenital amaurosis are targets for gene therapy pipelines, while ERTs continue to dominate the treatment of Gaucher, Pompe, and Fabry diseases. Pediatric indications are particularly prominent, as early diagnosis and intervention are crucial for reducing disease burden and improving quality of life.Biopharmaceutical companies specializing in rare diseases-such as BioMarin, Sarepta, Ultragenyx, and Spark Therapeutics-are leading clinical and commercial progress. These firms are often supported by nonprofit foundations, academic research networks, and patient advocacy groups that help drive trial recruitment and regulatory engagement. Global patient registries and rare disease consortia are increasingly being used to standardize data collection, validate endpoints, and justify accelerated approvals.
Geographically, North America and Europe remain dominant in drug development and regulatory activity, thanks to mature healthcare systems, funding ecosystems, and favorable policy frameworks. However, Asia-Pacific is seeing rapid momentum, particularly in gene editing research and biologics manufacturing capabilities, positioning it as a future growth frontier for rare genetic therapies.
What Is Driving Growth in the Rare Genetic Disorders Treatment Market?
The growth in the rare genetic disorders treatment market is driven by the convergence of scientific innovation, regulatory incentives, and increasing diagnosis rates enabled by genomic testing. As whole-genome sequencing becomes more accessible and affordable, previously undiagnosed or misdiagnosed conditions are being accurately classified, expanding the eligible treatment population. This diagnostic clarity is critical for precision medicine approaches like ASOs and gene therapy.Regulatory frameworks such as the Orphan Drug Act (USA), PRIME (EU), and Sakigake designation (Japan) are accelerating time-to-market for novel therapies. These policies reduce development risk, attract investment, and foster innovation through fast-track designations, smaller trial requirements, and longer exclusivity windows. Simultaneously, payer willingness to reimburse high-cost, high-impact treatments-often justified by long-term cost offsets-has strengthened the commercial viability of these therapies.
Collaborative ecosystems involving biotech firms, patient advocacy organizations, and regulatory bodies are ensuring that research is patient-centered and outcome-oriented. As digital health platforms and telemedicine expand, especially in post-approval patient monitoring and real-world evidence generation, the rare genetic disorders treatment market is positioned to evolve from niche to normalized care-redefining the future of personalized medicine.
Scope of the Report
The report analyzes the Rare Genetic Disorders Treatment market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Type (Biologics & Biosimilars, Non-Biologics); Distribution Channel (Hospitals Pharmacy, Retail Pharmacy, Online Pharmacy); Application (Cancer Application, Neurological Disorders Application, Cardiovascular Diseases Application, Metabolic Disorders Application, Hematology Diseases Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Biologics & Biosimilars segment, which is expected to reach US$171.6 Billion by 2030 with a CAGR of a 10.2%. The Non-Biologics segment is also set to grow at 14.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $40.8 Billion in 2024, and China, forecasted to grow at an impressive 11.3% CAGR to reach $47.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Rare Genetic Disorders Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Rare Genetic Disorders Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Rare Genetic Disorders Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alexion (AstraZeneca Rare Disease), Amicus Therapeutics, Arcturus Therapeutics, Beacon Therapeutics, BioMarin Pharmaceutical and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Rare Genetic Disorders Treatment market report include:
- Alexion (AstraZeneca Rare Disease)
- Amicus Therapeutics
- Arcturus Therapeutics
- Beacon Therapeutics
- BioMarin Pharmaceutical
- Bluebird Bio
- CRISPR Therapeutics
- Genomic startups (e.g., GeneQuine)
- PTC Therapeutics
- ProQR Therapeutics
- Rocket Pharmaceuticals
- Sarepta Therapeutics
- Spark Therapeutics
- Translate Bio (Sanofi)
- Tune Therapeutics
- Ultragenyx Pharmaceutical
- Vertex Pharmaceuticals
- Zygnema/Airna etc. (RNA-editing)
- Glycomine
- Calliditas Therapeutics
- Ipsen
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alexion (AstraZeneca Rare Disease)
- Amicus Therapeutics
- Arcturus Therapeutics
- Beacon Therapeutics
- BioMarin Pharmaceutical
- Bluebird Bio
- CRISPR Therapeutics
- Genomic startups (e.g., GeneQuine)
- PTC Therapeutics
- ProQR Therapeutics
- Rocket Pharmaceuticals
- Sarepta Therapeutics
- Spark Therapeutics
- Translate Bio (Sanofi)
- Tune Therapeutics
- Ultragenyx Pharmaceutical
- Vertex Pharmaceuticals
- Zygnema/Airna etc. (RNA-editing)
- Glycomine
- Calliditas Therapeutics
- Ipsen
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 170 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
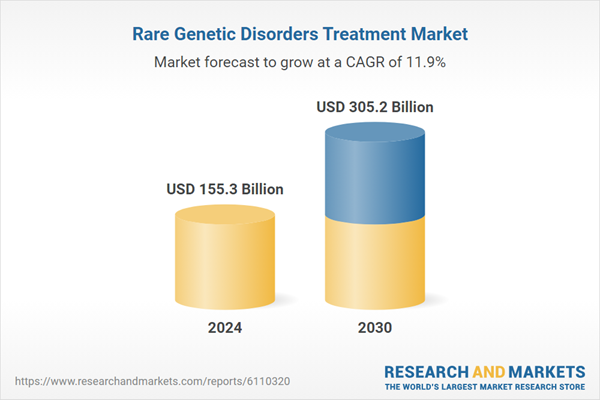
| Estimated Market Value ( USD | $ 155.3 Billion |
| Forecasted Market Value ( USD | $ 305.2 Billion |
| Compound Annual Growth Rate | 11.9% |
| Regions Covered | Global |