Global Lentiviral Vector Contract Development And Manufacturing Organizations (CDMO) Market - Key Trends & Drivers Summarized
Why Are Lentiviral CDMOs Gaining Strategic Significance in the Biotherapeutics Ecosystem?
Lentiviral vector (LVV) contract development and manufacturing organizations (CDMOs) are becoming vital enablers of the cell and gene therapy industry, supporting the scalable and compliant production of viral delivery systems used in ex vivo gene-modified cell therapies. Lentiviral vectors are commonly employed to deliver genetic payloads into non-dividing or slowly dividing cells, making them instrumental in the development of CAR-T cell therapies, gene-edited stem cells, and certain immunotherapy constructs. As the global pipeline of autologous and allogeneic cell therapies expands, biopharma companies are increasingly turning to CDMOs for lentiviral vector supply, given the complexity, regulatory demands, and infrastructure intensity of in-house viral manufacturing.The rise of approved and late-stage gene therapies, particularly in oncology and rare diseases, is placing acute pressure on lentiviral vector manufacturing capacity. Regulatory agencies mandate rigorous process validation, viral safety, and GMP-grade consistency, making CDMOs the preferred partners for early-stage development through commercial-scale production. These organizations offer end-to-end services including plasmid preparation, upstream and downstream vector production, analytical testing, and fill-finish under stringent regulatory frameworks such as FDA cGMP and EMA Annex 2. The expertise and infrastructure CDMOs bring to vector development are critical to accelerating timelines, de-risking scale-up, and navigating evolving quality requirements.
How Are Process Innovation and Modular Platforms Reshaping Lentiviral Manufacturing Services?
Lentiviral vector production presents unique challenges due to its reliance on transient transfection, low yields, and sensitivity to shear forces during purification. CDMOs are responding with innovations in process intensification, closed-system bioreactors, and chromatography-based purification workflows to improve yield, reduce contamination risks, and shorten batch cycles. Single-use technologies are now standard in upstream production, allowing rapid changeover, reduced validation time, and minimal cross-contamination-especially important for autologous therapies with patient-specific batches.Modular platform technologies are gaining traction, allowing CDMOs to offer standardized upstream/downstream workflows that can be rapidly adapted to different therapeutic candidates. These platforms often include HEK293-based producer cell lines, serum-free media, PEI-based transfection systems, and scalable filtration/purification units. Process development timelines are being compressed through the use of Design of Experiments (DoE), in-line analytics, and process analytical technology (PAT), which help identify critical quality attributes and optimal process parameters early in development.
Digitalization and automation are further streamlining operations. CDMOs are integrating digital twins, electronic batch records, and AI-assisted deviation tracking into their GMP suites to improve traceability, reproducibility, and regulatory readiness. These digital frameworks are especially important as regulatory bodies tighten oversight on vector integrity, empty/full capsid ratios, and adventitious agent testing. As vector manufacturing shifts from experimental to commercial-scale, these innovations are essential to support the reproducibility and cost-efficiency demanded by payer and regulatory ecosystems.
Which Client Segments and Therapeutic Modalities Are Driving CDMO Engagement?
Biotech firms-particularly early- and mid-stage developers-represent the primary client base for lentiviral CDMOs, as they often lack in-house GMP manufacturing capabilities. These companies seek CDMO partners to handle preclinical vector supply, IND-enabling process development, and clinical-grade manufacturing, allowing them to focus on therapeutic design, regulatory strategy, and fundraising. Late-stage biotech firms and Big Pharma companies are also increasing their reliance on CDMOs to access scalable capacity, regional manufacturing footprints, and redundancy planning for commercial launches.Therapeutic areas driving lentiviral demand include oncology (notably CAR-T therapies), hematologic disorders (e.g., sickle cell disease, β-thalassemia), and rare genetic diseases such as Wiskott-Aldrich syndrome and adrenoleukodystrophy. The rise of in vivo lentiviral applications-although still early-could expand CDMO engagement further, especially if formulation and targeting technologies advance to support direct administration. Additionally, allogeneic and off-the-shelf cell therapies, which require large-scale vector production, are creating demand for commercial-ready facilities with large-volume bioreactors, cryopreservation systems, and robust QC frameworks.
Academic institutions and hospital-led clinical trials also represent a niche but growing client group, especially in geographies where public funding supports translational research. These groups often partner with regional CDMOs for GMP vector production to bridge the gap between laboratory research and human studies. Non-profit consortia, military biodefense units, and global health agencies are further diversifying the demand base, particularly for scalable platforms that can address global diseases through gene-based interventions.
What Is Fueling Growth in the Lentiviral Vector CDMO Market Worldwide?
The growth in the global lentiviral vector CDMO market is driven by several factors, including the rapid expansion of gene-modified cell therapies, increasing regulatory stringency, and sustained venture and biopharma investment in gene therapy pipelines. As more gene and cell therapies enter late-stage clinical development and gain market authorization, the demand for high-quality, scalable lentiviral vectors is rising sharply. CDMOs provide critical expertise and infrastructure to support GMP production, reduce time to market, and maintain regulatory compliance through all development phases.Manufacturing bottlenecks and the high fixed cost of vector facilities are pushing developers toward outsourcing, especially in the pre-commercial phase. CDMOs are capitalizing on this by expanding capacity through greenfield investments, acquisitions, and technology licensing. Globalization of clinical trials is also encouraging CDMOs to establish multipoint manufacturing hubs in North America, Europe, and Asia to support regional regulatory compliance and reduce logistical complexity. Governments are supporting this expansion through innovation grants, public-private partnerships, and expedited approvals for ATMP-related infrastructure.
Strategic collaborations between vector CDMOs, cell therapy developers, and technology providers are fostering innovation across the value chain. Standardization of platform technologies, digital infrastructure, and regulatory guidance for viral vectors are also reducing development friction. With a robust pipeline of gene therapy assets and growing regulatory momentum, lentiviral CDMOs are poised for sustained demand growth, making them indispensable players in the next phase of personalized and regenerative medicine.
Scope of the Report
The report analyzes the Lentiviral Vector Contract Development and Manufacturing Organizations market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Component (Lentiviral Promoter, Lentiviral Fusion Tags, Lentivirus Packaging Systems, Other Components); Disease Indication (Cancer, Genetic Disorders, Infectious Diseases, Veterinary Disease, Other Disease Indications); End-User (Pharmaceutical & Biotechnology Company End-User, Academic Research End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Lentiviral Promoter segment, which is expected to reach US$81.7 Million by 2030 with a CAGR of a 6.2%. The Lentiviral Fusion Tags segment is also set to grow at 3.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $42.7 Million in 2024, and China, forecasted to grow at an impressive 7.8% CAGR to reach $41.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lentiviral Vector Contract Development and Manufacturing Organizations Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lentiviral Vector Contract Development and Manufacturing Organizations Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lentiviral Vector Contract Development and Manufacturing Organizations Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AGC Biologics, Aldevron, Andelyn Biosciences, Batavia Biosciences, BioReliance (Merck KGaA) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Lentiviral Vector Contract Development and Manufacturing Organizations market report include:
- AGC Biologics
- Aldevron
- Andelyn Biosciences
- Batavia Biosciences
- BioReliance (Merck KGaA)
- BioVec Pharma
- BOEHRINGER INGELHEIM BioXcellence
- Brammer Bio (Thermo Fisher Scientific)
- Cell and Gene Therapy Catapult
- Charles River Laboratories
- Creative Biogene
- Eurofins CDMO
- Genezen
- IDT Biologika
- Lonza
- Novasep (part of Sartorius)
- Oxford Biomedica
- Sartorius Stedim BioOutsource
- Thermo Fisher Scientific
- Vibalogics
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AGC Biologics
- Aldevron
- Andelyn Biosciences
- Batavia Biosciences
- BioReliance (Merck KGaA)
- BioVec Pharma
- BOEHRINGER INGELHEIM BioXcellence
- Brammer Bio (Thermo Fisher Scientific)
- Cell and Gene Therapy Catapult
- Charles River Laboratories
- Creative Biogene
- Eurofins CDMO
- Genezen
- IDT Biologika
- Lonza
- Novasep (part of Sartorius)
- Oxford Biomedica
- Sartorius Stedim BioOutsource
- Thermo Fisher Scientific
- Vibalogics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 378 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
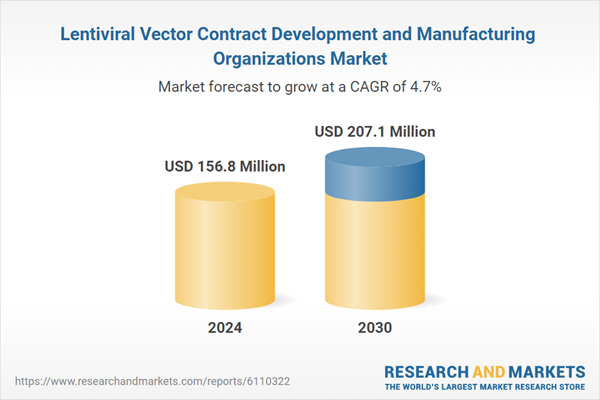
| Estimated Market Value ( USD | $ 156.8 Million |
| Forecasted Market Value ( USD | $ 207.1 Million |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |