Global Small Molecules CDMO Market - Key Trends & Drivers Summarized
Why Are Small Molecule CDMOs Now Critical Pillars in Global Pharma Supply Chains?
Small Molecule Contract Development and Manufacturing Organizations (CDMOs) have become integral to the pharmaceutical value chain, offering end-to-end services for drug substance (API) and drug product (formulation) development. As pharmaceutical companies streamline operations, reduce time-to-market, and respond to cost and capacity pressures, they are increasingly outsourcing complex small molecule projects to specialized CDMOs. These partners bring deep process chemistry expertise, global regulatory understanding, and advanced infrastructure - factors crucial for scaling from preclinical R&D to commercial manufacturing.Small molecules still account for the vast majority of drugs on the market, particularly in areas like oncology, cardiology, CNS disorders, and infectious diseases. Their relatively stable formulations, well-established regulatory pathways, and oral bioavailability make them the dominant therapeutic modality even amid the biologics boom. Consequently, CDMOs with robust small molecule capabilities remain highly sought after - not only by large pharmaceutical companies but also by mid-sized firms and biotech startups needing scalable, GMP-compliant infrastructure to bring drug candidates to market.
How Are Technology and Regulatory Complexity Shaping CDMO Service Models?
The scope of services provided by modern CDMOs has evolved from basic contract manufacturing to comprehensive, integrated offerings. These include process development, analytical method validation, solid-state chemistry, regulatory submission support, formulation design, and packaging solutions. Advanced CDMOs are investing in continuous manufacturing, green chemistry, and automation technologies to reduce cycle times, environmental footprint, and cost per batch - an advantage especially for high-volume or niche indication drugs.Simultaneously, the regulatory environment is growing more intricate. Agencies like the FDA, EMA, and PMDA are requiring deeper process transparency, data integrity, and quality-by-design (QbD) approaches. CDMOs must meet these expectations through robust digital documentation systems, real-time batch analytics, and stringent quality systems across facilities. As global regulatory harmonization gains traction, multinational clients are seeking CDMOs with cross-border compliance expertise and multi-site regulatory accreditations - spurring a consolidation trend among high-performing vendors.
Where Is Demand for Small Molecule CDMOs Surging the Most?
The fastest-growing demand for small molecule CDMOs is coming from emerging biopharma, which now accounts for the majority of early-stage drug development activity worldwide. These companies rely heavily on outsourced manufacturing due to lack of in-house capabilities. Oncology and rare disease segments - many of which are dominated by small molecules - are contributing significant project volume, especially for Phase I and II clinical trials. As patent cliffs approach for blockbuster drugs, large pharma companies are also outsourcing generics and lifecycle extension work, including reformulations and fixed-dose combinations.Regionally, North America remains the largest market, followed closely by Europe, where regulatory stringency and high-quality standards elevate demand for premium CDMO partners. However, Asia-Pacific is emerging as a key manufacturing hub, particularly in India and China, driven by competitive cost structures, expanding GMP compliance, and growing talent pools. There is also a notable rise in onshoring initiatives in the US and Europe, prompting CDMOs to expand local capacity to mitigate geopolitical risks and ensure supply chain continuity.
The Growth in the Small Molecules CDMO Market Is Driven by Several Factors…
The market is expanding due to sustained demand for scalable, high-quality outsourcing solutions across the drug development lifecycle. The dominance of small molecule drugs in global pipelines, particularly for chronic and emerging diseases, ensures strong underlying demand. Rising R&D investments by emerging biopharma, coupled with limited internal manufacturing resources, are fueling CDMO dependency. Technological advancements in continuous manufacturing, high-potency compound handling, and digital process analytics are increasing the value proposition of CDMOs. Additionally, rising regulatory complexity, GMP compliance pressures, and supply chain globalization are prompting pharmaceutical companies to form long-term, strategic outsourcing partnerships. Regional growth in Asia-Pacific, combined with capacity expansions and M&A activity among leading CDMOs, is further amplifying market momentum across clinical and commercial phases.Scope of the Report
The report analyzes the Small Molecules Contract Development and Manufacturing Organization market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Small Molecule API, Small Molecule Drug Products); Stage (Preclinical Stage, Clinical Stage, Commercial Stage); Therapeutic Area (Oncology Therapeutic Area, Cardiovascular Diseases Therapeutic Area, Respiratory Disorders Therapeutic Area, Neurology Therapeutic Area, Metabolic Disorders Therapeutic Area, Other Therapeutic Areas); End-Use (Pharma & Biotech Companies End-Use, Research Institutes & Academics End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Small Molecule API segment, which is expected to reach US$150.3 Billion by 2030 with a CAGR of a 4.4%. The Small Molecule Drug Products segment is also set to grow at 7.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $47.7 Billion in 2024, and China, forecasted to grow at an impressive 8.4% CAGR to reach $47.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Small Molecules Contract Development and Manufacturing Organization Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Small Molecules Contract Development and Manufacturing Organization Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Small Molecules Contract Development and Manufacturing Organization Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ajinomoto Bio-Pharma Services, Aenova Group, Alcami Corporation, Amgen Inc., Aurigene Pharmaceutical Services Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Small Molecules Contract Development and Manufacturing Organization market report include:
- Ajinomoto Bio-Pharma Services
- Aenova Group
- Alcami Corporation
- Amgen Inc.
- Aurigene Pharmaceutical Services Ltd.
- Bellen Chemistry
- Boehringer Ingelheim
- Cambrex Corporation
- Catalent, Inc.
- CordenPharma International
- CoreRx, Inc.
- Delpharm
- Eurofins Scientific
- Hovione
- Labcorp Drug Development
- Lonza Group Ltd.
- Pace Analytical Services, LLC
- Piramal Pharma Solutions
- WuXi AppTec Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ajinomoto Bio-Pharma Services
- Aenova Group
- Alcami Corporation
- Amgen Inc.
- Aurigene Pharmaceutical Services Ltd.
- Bellen Chemistry
- Boehringer Ingelheim
- Cambrex Corporation
- Catalent, Inc.
- CordenPharma International
- CoreRx, Inc.
- Delpharm
- Eurofins Scientific
- Hovione
- Labcorp Drug Development
- Lonza Group Ltd.
- Pace Analytical Services, LLC
- Piramal Pharma Solutions
- WuXi AppTec Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 479 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
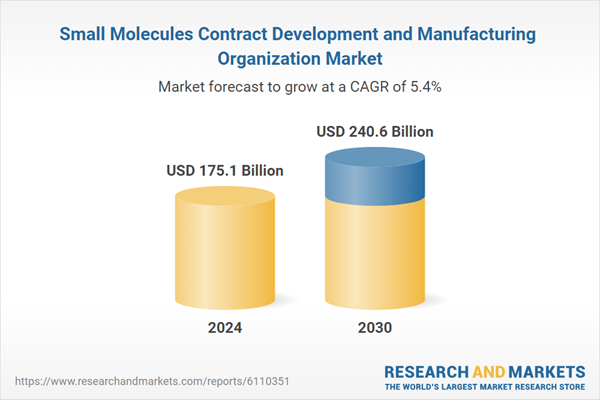
| Estimated Market Value ( USD | $ 175.1 Billion |
| Forecasted Market Value ( USD | $ 240.6 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |