Global Lymphatic Malformations Market - Key Trends & Drivers Summarized
Why Is There Renewed Clinical and Research Focus on Lymphatic Malformations?
Lymphatic malformations (LMs) are rare congenital vascular anomalies resulting from abnormal development of the lymphatic system, leading to cystic or spongy masses that can appear in the neck, axilla, mediastinum, or other body parts. They are typically diagnosed in infancy or early childhood, but adult-onset LMs are increasingly recognized. Although benign, their potential to compress vital structures, cause disfigurement, and impair quality of life drives significant medical interest. Historically managed through surgical excision or sclerotherapy, treatment paradigms are shifting due to advances in molecular understanding and targeted therapeutics.Rising diagnostic awareness, improved imaging technologies, and the expanding availability of multidisciplinary vascular anomaly clinics are contributing to early detection and better classification of LMs. MRI, ultrasound, and lymphangiography allow clinicians to distinguish between microcystic, macrocystic, and mixed-type LMs, guiding treatment selection more precisely. Recent genetic studies have identified PIK3CA mutations as a frequent cause of complex lymphatic anomalies, linking LMs to the broader spectrum of PIK3CA-related overgrowth syndromes (PROS). This molecular insight is catalyzing targeted drug development and clinical trials for precision treatment of LMs.
How Are Therapeutic Options Evolving Beyond Conventional Surgical Approaches?
Traditionally, surgery and sclerotherapy have been the cornerstone of LM management, especially for macrocystic lesions. However, recurrence risks, incomplete resection, and procedural complications have driven the search for less invasive and more durable treatment modalities. Minimally invasive procedures, such as image-guided percutaneous sclerotherapy using agents like bleomycin, doxycycline, and OK-432, are gaining favor due to reduced morbidity and shorter recovery times. However, response rates vary based on lesion type, location, and patient age.Systemic pharmacological therapies are gaining traction, especially for diffuse or refractory LMs. The use of sirolimus (rapamycin), an mTOR inhibitor, has shown promise in reducing LM volume and alleviating symptoms by targeting abnormal cell proliferation and lymphangiogenesis pathways. Early-phase clinical trials for next-generation PI3K inhibitors are underway, seeking to address the underlying genetic drivers of complex lymphatic anomalies. Compassionate use protocols and off-label regimens are being carefully evaluated for long-term efficacy and safety.
Emerging therapies such as radiofrequency ablation, laser photocoagulation, and cryotherapy are also being explored for localized lesions. Multimodal treatment approaches-combining surgery, interventional radiology, and pharmacotherapy-are now standard in leading academic centers. Patient-specific treatment plans, genetic counseling, and longitudinal follow-up are forming the basis of precision LM management, supported by international registries and collaborative research networks focused on vascular anomalies.
Which Clinical Practices and Geographic Markets Are Influencing Treatment Trends?
Tertiary hospitals, children’s specialty hospitals, and vascular anomaly clinics remain the primary hubs for LM treatment due to the need for interdisciplinary expertise. Pediatricians, dermatologists, interventional radiologists, geneticists, and craniofacial surgeons work collaboratively to design and deliver care. North America and Western Europe lead in advanced care models, offering access to sclerotherapy, targeted drugs, and comprehensive follow-up. Centers in the U.S., Canada, Germany, and France have established themselves as global leaders in LM research and clinical innovation.Asia-Pacific, particularly countries like Japan, South Korea, and China, is witnessing increased diagnosis rates and clinical awareness. The use of sclerosing agents like OK-432 (Picibanil), originally developed in Japan, has gained widespread adoption across East Asia. Latin America and Africa face challenges in access to specialized care, but telemedicine and international clinical collaborations are expanding treatment access. Global efforts by organizations such as the International Society for the Study of Vascular Anomalies (ISSVA) are also standardizing diagnostic criteria and therapeutic approaches across borders.
Patient advocacy groups and rare disease alliances are playing a critical role in promoting clinical trials, publishing educational resources, and supporting affected families. Increased insurance coverage for off-label therapies, orphan drug designations, and regulatory incentives are helping expand the therapeutic arsenal. Medical training programs are also incorporating LM-focused modules to improve early detection and interdisciplinary coordination among practitioners.
What Is Fueling Growth in the Lymphatic Malformations Market Globally?
The growth in the global lymphatic malformations market is driven by several factors, including rising awareness of rare vascular disorders, improved diagnostic imaging capabilities, and the emergence of targeted pharmacological therapies. The molecular characterization of LMs as part of the PIK3CA mutation spectrum has unlocked new research avenues and drug development programs, attracting the interest of both academic institutions and biopharmaceutical companies.The growing demand for minimally invasive procedures and personalized treatment strategies is expanding the role of advanced interventional radiology and systemic therapy. Healthcare systems in high-income countries are investing in dedicated vascular anomaly units, while low- and middle-income regions are benefiting from increasing telehealth penetration and international clinical collaborations. Regulatory designations for rare disease treatments and inclusion of LM therapies in pediatric clinical trial frameworks are further encouraging innovation.
Advancements in precision diagnostics, combined with patient-centric care models, are reshaping clinical practices and driving long-term market growth. As more evidence emerges on the safety and efficacy of novel treatments-particularly mTOR and PI3K inhibitors-the therapeutic landscape for lymphatic malformations is expected to evolve significantly. The convergence of medical research, digital health platforms, and global rare disease networks positions the LM market for sustainable growth and improved patient outcomes in the coming years.
Scope of the Report
The report analyzes the Lymphatic Malformations market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Diagnosis Application, Treatment Application); End-User (Hospitals End-User, Clinics End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Diagnosis Application segment, which is expected to reach US$158.8 Million by 2030 with a CAGR of a 3.7%. The Treatment Application segment is also set to grow at 6.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $51.9 Million in 2024, and China, forecasted to grow at an impressive 7.1% CAGR to reach $48.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lymphatic Malformations Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lymphatic Malformations Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lymphatic Malformations Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alexion Pharmaceuticals, Amryt Pharma, Arch Therapeutics, Bayer AG, Boston Scientific and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Lymphatic Malformations market report include:
- Alexion Pharmaceuticals
- Amryt Pharma
- Arch Therapeutics
- Bayer AG
- Boston Scientific
- BTG International
- Cook Medical
- Elekta AB
- F. Hoffmann-La Roche Ltd
- Fresenius Kabi
- GlaxoSmithKline (GSK)
- Hamamatsu Photonics
- InSightec
- Johnson & Johnson
- Medtronic
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sobi (Swedish Orphan Biovitrum)
- Takeda Pharmaceutical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alexion Pharmaceuticals
- Amryt Pharma
- Arch Therapeutics
- Bayer AG
- Boston Scientific
- BTG International
- Cook Medical
- Elekta AB
- F. Hoffmann-La Roche Ltd
- Fresenius Kabi
- GlaxoSmithKline (GSK)
- Hamamatsu Photonics
- InSightec
- Johnson & Johnson
- Medtronic
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sobi (Swedish Orphan Biovitrum)
- Takeda Pharmaceutical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 277 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
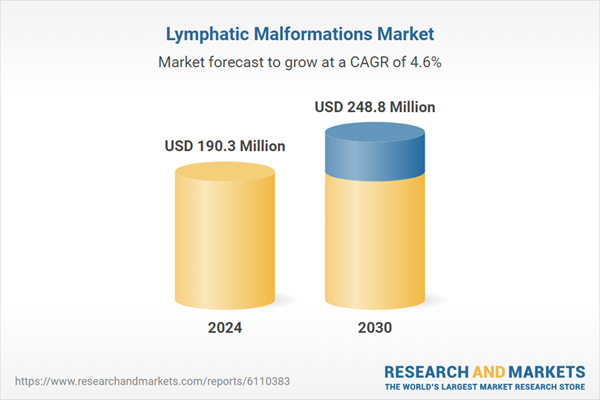
| Estimated Market Value ( USD | $ 190.3 Million |
| Forecasted Market Value ( USD | $ 248.8 Million |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |