Global Venous Procedure Devices Market - Key Trends & Drivers Summarized
Why Are Venous Procedure Devices Experiencing Elevated Demand Across Multiple Specialties?
Venous procedure devices, encompassing technologies used in the treatment of chronic venous insufficiency (CVI), deep vein thrombosis (DVT), varicose veins, and venous ulcers, are experiencing a demand resurgence due to demographic aging, sedentary lifestyles, and rising incidence of vascular disorders. These devices include radiofrequency and laser ablation catheters, sclerotherapy kits, venous stents, thrombectomy devices, and compression systems, all of which support minimally invasive interventions aimed at improving venous return and preventing complications. Clinical uptake is expanding across vascular surgery, interventional radiology, and dermatology practices.A particularly strong growth driver is the rising prevalence of venous thromboembolism (VTE), a leading cause of preventable death in hospitals worldwide. The increasing use of diagnostic imaging, awareness of post-thrombotic syndrome (PTS), and improved referral pathways are expanding the treatable patient pool. Parallel to this, advancements in office-based procedures and outpatient settings are driving demand for portable, image-guided ablation systems and disposable catheter kits. Consumer-driven aesthetics and quality-of-life concerns are also pushing demand for cosmetic varicose vein removal, further broadening the clinical landscape for venous devices.
How Are Innovations in Device Design Enhancing Procedural Efficiency and Outcomes?
Technology evolution in venous procedure devices is focused on improving patient comfort, reducing recurrence rates, and enabling rapid recovery. Next-generation endovenous ablation systems use bipolar radiofrequency and fiber-delivered laser energy for precision thermal closure of incompetent veins, with integrated cooling and feedback mechanisms to minimize thermal injury. Mechanochemical ablation (MOCA) systems, which combine mechanical irritation with sclerosant infusion, are gaining adoption for their non-thermal, anesthesia-free benefits and suitability in tortuous venous anatomy.Venous stents are witnessing a transformation, with nitinol-based self-expanding designs offering improved patency, radial force, and conformity to the venous anatomy. Thrombus management has advanced through catheter-directed thrombolysis (CDT), ultrasonic-assisted thrombolysis, and aspiration thrombectomy, offering options tailored to clot burden, acuity, and bleeding risk. The integration of real-time imaging and AI-powered navigation systems is helping clinicians better target treatment zones and minimize collateral tissue damage. Moreover, the increased availability of single-use, sterile kits is streamlining workflow and reducing infection risk across care settings.
Where Is Utilization of Venous Devices Rising and What Patient Segments Are Being Prioritized?
Venous procedure devices are witnessing widespread adoption in North America and Europe, where aging populations and robust reimbursement frameworks support high procedure volumes. In the U.S., office-based labs (OBLs) and ambulatory surgical centers (ASCs) are key users, supported by CMS policies favoring outpatient interventions. Hospitals are also using advanced thrombectomy systems in acute stroke prevention workflows for patients presenting with DVT or pulmonary embolism. In Europe, countries such as Germany, the UK, and France have standardized protocols for CVI and VTE, resulting in consistent device demand.Emerging markets in Latin America, the Middle East, and Asia-Pacific are seeing a rise in venous procedures driven by increased cardiovascular screening, improved medical tourism infrastructure, and growing awareness among primary care physicians. In India and Southeast Asia, cost-effective venous ablation kits and foam sclerotherapy systems are enabling large-scale treatment campaigns for underserved rural populations. A key focus is on women over 40, a group disproportionately affected by varicose veins and CVI due to hormonal changes, occupational standing, and pregnancy-related venous stress. This demographic, along with high-risk post-surgical and oncology patients, constitutes a high-priority segment.
What Is Driving Growth in the Global Venous Procedure Devices Market?
The growth in the venous procedure devices market is driven by several factors, including the increasing burden of venous diseases, the shift toward outpatient minimally invasive interventions, and the advancement of technology that improves both efficacy and safety. Healthcare providers are prioritizing early intervention in chronic venous disorders to reduce hospitalizations, improve mobility, and prevent life-threatening complications like pulmonary embolism. The global rise in aging populations, diabetes, and obesity are also significant underlying drivers.Device miniaturization, enhanced energy delivery platforms, and growing patient preference for minimally disruptive treatment are pushing the industry toward continuous innovation. Favorable reimbursement policies, cross-specialty collaboration, and the proliferation of hybrid vascular centers are expanding procedural access. Additionally, training initiatives and CME programs are equipping physicians in emerging markets with the skills and protocols to deliver advanced venous care. With procedural volumes rising and new patient populations being identified through digital screening tools, the venous procedure devices market is set for sustained expansion across clinical, geographic, and consumer dimensions.
Scope of the Report
The report analyzes the Venous Procedure Devices market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Catheters, Guidewires, Other Product Types); Disease (Vascular Disease, Cancer Disease); Application (Leg Application, Chest Application, Abdomen Application, Arm Application); End-User (Hospitals End-User, Ambulatory Surgery Centers End-User, Specialty Clinics End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Catheters segment, which is expected to reach US$2.3 Billion by 2030 with a CAGR of a 6.7%. The Guidewires segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $718.5 Million in 2024, and China, forecasted to grow at an impressive 9.2% CAGR to reach $749.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Venous Procedure Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Venous Procedure Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Venous Procedure Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, AngioDynamics, Asahi Kasei Medical, B. Braun Melsungen AG, Baxter International and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Venous Procedure Devices market report include:
- Abbott Laboratories
- AngioDynamics
- Asahi Kasei Medical
- B. Braun Melsungen AG
- Baxter International
- Bayer (vascular division)
- Becton Dickinson (BD)
- Biolitec AG
- Boston Scientific
- Boston Scientific Corporation
- Candela Corporation
- Cardinal Health
- Cook Medical
- Cordis
- Eufoton
- Fresenius Medical Care
- IC U Medical
- Inari Medical
- LeMaitre Vascular
- Lumenis Be Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AngioDynamics
- Asahi Kasei Medical
- B. Braun Melsungen AG
- Baxter International
- Bayer (vascular division)
- Becton Dickinson (BD)
- Biolitec AG
- Boston Scientific
- Boston Scientific Corporation
- Candela Corporation
- Cardinal Health
- Cook Medical
- Cordis
- Eufoton
- Fresenius Medical Care
- IC U Medical
- Inari Medical
- LeMaitre Vascular
- Lumenis Be Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 474 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
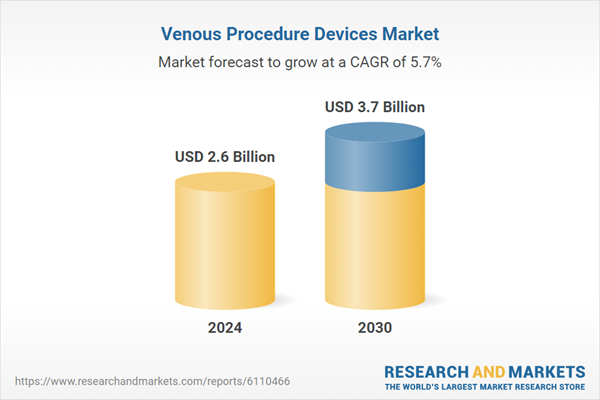
| Estimated Market Value ( USD | $ 2.6 Billion |
| Forecasted Market Value ( USD | $ 3.7 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |