Global Radial Artery Compression Devices Market - Key Trends & Drivers Summarized
Why Are Radial Artery Compression Devices Becoming Indispensable in Vascular Procedures?
Radial artery compression devices (RACDs) are now a cornerstone in post-procedural hemostasis management following transradial access interventions such as coronary angiography, angioplasty, or cardiac catheterization. These devices replace traditional manual compression and pressure dressings, offering superior control, patient comfort, and reduced vascular complications. As transradial access becomes the preferred method in interventional cardiology, driven by its lower bleeding risks and shorter recovery times compared to femoral access, demand for advanced compression solutions is accelerating across both developed and emerging markets.The core functionality of RACDs lies in their ability to provide controlled, adjustable pressure over the puncture site to prevent bleeding while preserving radial artery patency. Innovations have led to the development of pneumatic wristbands, band-based compression systems, and even automated digital pressure control devices. These are now widely used not only in tertiary hospitals but also in outpatient and ambulatory care settings where procedural volume is rising. The broader shift toward same-day discharge and minimally invasive cardiac care has further amplified the need for efficient, safe, and patient-friendly hemostasis solutions.
What Technological Advancements Are Improving Device Safety and Usability?
Modern RACDs have evolved considerably in design and functionality. Contemporary systems incorporate transparent pressure pads for visual site monitoring, quick-release valves for pressure titration, and anatomical contouring for customized fit. These features reduce the risk of complications such as radial artery occlusion (RAO), hematoma formation, or excessive ischemia, which can arise from improper or prolonged compression. Smart variants are integrating pressure sensors and feedback systems to ensure optimal compression levels, allowing clinicians to transition from empirical to data-guided application protocols.In addition to safety, usability is a key driver of adoption. Devices with single-hand application, color-coded pressure zones, and user-friendly adjustment mechanisms are improving procedural throughput and reducing nursing workload. Reusability and disposability considerations are also influencing device selection, with many facilities choosing cost-effective single-use systems for infection control compliance. The emergence of hybrid devices capable of integrating hemostasis with radial artery preservation protocols-such as patent hemostasis-represents a major step forward in reducing late-stage complications.
Regulatory agencies such as the FDA and EMA now recognize RACDs as Class II devices, and global standardization efforts are underway to harmonize usage protocols. This has increased trust among healthcare providers and catalyzed broader institutional adoption. Furthermore, real-world evidence from observational studies and clinical trials consistently shows improved outcomes and patient satisfaction, reinforcing the value proposition of RACDs over conventional compression methods.
Which Healthcare Settings and Geographies Are Driving Market Expansion?
The global expansion of catheter-based cardiovascular diagnostics and interventions is directly linked to RACD adoption. Hospitals and cardiac centers are the primary end-users, but ambulatory surgical centers (ASCs) and mobile cardiac units are increasingly investing in these devices to support efficient workflows and fast patient turnover. In regions where healthcare is moving toward value-based care, RACDs offer measurable improvements in recovery time, procedural safety, and nursing efficiency-all of which contribute to better resource utilization.North America, particularly the United States, represents a mature market with high procedural volume and a favorable reimbursement ecosystem for radial access procedures. Europe is also well established, with widespread adherence to radial-first protocols in cardiology departments. However, the most dynamic growth is now occurring in Asia-Pacific, where countries like India, China, and South Korea are scaling up interventional cardiology programs in response to surging cardiovascular disease burdens. Latin America and parts of the Middle East are emerging as secondary growth hubs due to government investments in public cardiology infrastructure.
Education and training initiatives are playing a catalytic role. Several institutions now mandate formal radial access training for interventional cardiologists, and device manufacturers are offering integrated hemostasis kits bundled with training modules and support services. This ecosystem approach is fostering adoption not only in top-tier hospitals but also in secondary care facilities where radial access was historically underutilized.
What Is Driving Growth in the Global Radial Artery Compression Devices Market?
The growth in the global radial artery compression devices market is driven by the widespread shift toward transradial access in cardiovascular and neurovascular procedures, combined with advancements in hemostasis technology. As radial access becomes the standard of care globally, RACDs are no longer optional but a critical component of post-procedural success. Their role in reducing complications, expediting discharge, and enhancing patient comfort is directly aligned with the goals of modern cardiovascular care.Technological innovation is another growth accelerator. Devices with real-time pressure monitoring, ergonomic design, and evidence-based patent hemostasis features are gaining clinical preference. As healthcare providers aim to optimize outcomes and minimize readmission rates, RACDs are proving their value as both preventive and efficiency-enhancing tools. Their cost-effectiveness and reduced nursing burden also make them attractive in value-conscious healthcare systems.
Future growth is expected to stem from procedural diversification-such as their use in transradial neurointerventions and peripheral artery disease diagnostics-as well as geographic expansion into mid-income economies scaling up cardiac infrastructure. With the convergence of clinical best practices, technological maturity, and supportive reimbursement structures, radial artery compression devices are poised for sustained demand across the cardiovascular care continuum.
Scope of the Report
The report analyzes the Radial Artery Compression Devices market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Band / Strap-based Device, Knob-based Device, Plate-based Device); Application (Surgical Intervention Application, Diagnostics Application); End-Use (Hospitals End-Use, Ambulatory Surgery Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Band / Strap-based Device segment, which is expected to reach US$152.9 Million by 2030 with a CAGR of a 4.3%. The Knob-based Device segment is also set to grow at 5.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $56.7 Million in 2024, and China, forecasted to grow at an impressive 7.8% CAGR to reach $54.7 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Radial Artery Compression Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Radial Artery Compression Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Radial Artery Compression Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Advanced Vascular Dynamics, AngioDynamics, Inc., Beijing Demax Medical Technology, Becton, Dickinson & Company (BD) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Radial Artery Compression Devices market report include:
- Abbott Laboratories
- Advanced Vascular Dynamics
- AngioDynamics, Inc.
- Beijing Demax Medical Technology
- Becton, Dickinson & Company (BD)
- Cardinal Health, Inc.
- Comed B.V. / ConMed B.V.
- Forge Medical Inc.
- Kardia Medical
- Lepu Medical Technology (Beijing)
- Medtronic plc
- Merit Medical Systems, Inc.
- Nipro Corporation
- Scitech Products Ltd.
- Semler Technologies Inc.
- Smiths Medical
- Teleflex Incorporated
- Terumo Corporation
- TZ Medical, Inc.
- Vascular Solutions (Teleflex)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Advanced Vascular Dynamics
- AngioDynamics, Inc.
- Beijing Demax Medical Technology
- Becton, Dickinson & Company (BD)
- Cardinal Health, Inc.
- Comed B.V. / ConMed B.V.
- Forge Medical Inc.
- Kardia Medical
- Lepu Medical Technology (Beijing)
- Medtronic plc
- Merit Medical Systems, Inc.
- Nipro Corporation
- Scitech Products Ltd.
- Semler Technologies Inc.
- Smiths Medical
- Teleflex Incorporated
- Terumo Corporation
- TZ Medical, Inc.
- Vascular Solutions (Teleflex)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 374 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
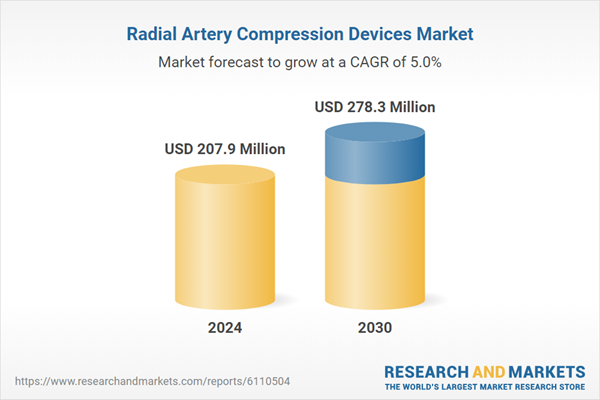
| Estimated Market Value ( USD | $ 207.9 Million |
| Forecasted Market Value ( USD | $ 278.3 Million |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |