Global Wearable Cardioverter Defibrillators Market - Key Trends & Drivers Summarized
Why Are Wearable Cardioverter Defibrillators Becoming Essential in Sudden Cardiac Arrest Prevention?
The global incidence of sudden cardiac arrest (SCA) is driving a significant rise in the adoption of wearable cardioverter defibrillators (WCDs), as patients and physicians increasingly seek non-invasive, interim protection against life-threatening arrhythmias. Unlike implantable cardioverter defibrillators (ICDs), WCDs are external devices designed to monitor the heart continuously and deliver a shock if a life-threatening ventricular arrhythmia is detected. These devices are particularly beneficial for patients who are temporarily at high risk of SCA but are not immediate candidates for implantable devices, such as those recovering from myocardial infarction, awaiting heart transplant, or undergoing evaluation for ICD candidacy. The non-invasive nature of WCDs reduces the risk of procedural complications and offers a safer bridge during periods of clinical uncertainty. Additionally, WCDs empower patients to maintain mobility and quality of life while under constant cardiac surveillance, providing peace of mind and clinical oversight without hospitalization. The real-time data collected by these devices also assists physicians in treatment planning and risk stratification. Growing awareness among cardiologists about the advantages of WCDs in preventing sudden death in high-risk populations has increased prescriptions, especially in regions where heart disease is a leading cause of mortality. As healthcare systems globally shift toward proactive and preventive care, WCDs are emerging as an indispensable tool in reducing avoidable cardiac fatalities.How Is Technology Advancing the Reliability and User-Friendliness of WCDs?
Recent technological advancements are transforming wearable cardioverter defibrillators into more sophisticated, comfortable, and user-friendly medical devices, thereby accelerating their adoption across various patient groups. Early generations of WCDs were often bulky and less intuitive, but modern models now feature lightweight designs, improved fit, and skin-friendly materials that support long-term wear without discomfort. The integration of wireless communication technologies allows real-time data transmission to healthcare providers, enabling remote monitoring and faster clinical decision-making. Enhanced ECG algorithms have significantly reduced false alarms and improved the precision of arrhythmia detection, ensuring that shocks are delivered only when truly necessary. Some devices now incorporate voice prompts and automated instructions, simplifying patient response during emergencies and improving survival outcomes. Additionally, battery life improvements and automated self-check features have increased operational reliability and reduced maintenance burdens on users. Cloud-based platforms paired with WCDs enable physicians to track patient adherence, monitor arrhythmia trends, and intervene when needed, fostering a more proactive care approach. Innovations in miniaturization and sensor technologies are also opening the door for more discreet form factors that improve patient compliance. These continuous improvements in design and functionality are making WCDs not only more clinically effective but also more acceptable and manageable for everyday use, which is critical for long-term protection and patient adherence.What Factors Are Expanding the Use of WCDs Across Patient Populations and Regions?
The clinical and demographic landscape surrounding cardiovascular disease is broadening the scope of wearable cardioverter defibrillator usage across patient populations and geographic regions. Traditionally prescribed for post-myocardial infarction or heart failure patients, WCDs are now being explored for use in younger patients with genetic conditions such as hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, or long QT syndrome. These high-risk groups may not immediately qualify for ICD implantation but still face significant arrhythmic threats, making temporary wearable protection a critical intervention. Emerging markets in Asia, Latin America, and parts of Eastern Europe are witnessing increased demand as cardiovascular disease prevalence rises due to urbanization, dietary changes, and aging populations. In these regions, WCDs offer a cost-effective alternative to ICDs, especially where healthcare infrastructure may limit access to surgical procedures. Additionally, healthcare providers are becoming more aware of the need to reduce hospital stays and readmission rates, which is prompting earlier discharge of patients with a WCD as part of their transition-of-care plans. Insurance coverage and reimbursement policies are also evolving to accommodate WCDs, making them more accessible in both public and private healthcare systems. Academic research and clinical trials are expanding evidence-based use cases, further validating the role of WCDs in diverse cardiac risk scenarios. This expansion in clinical applicability and global reach is making WCDs a more integral component of comprehensive cardiac care pathways.What Are the Core Drivers Propelling the Growth of the Wearable Cardioverter Defibrillator Market?
The growth in the wearable cardioverter defibrillator market is driven by several key factors rooted in clinical demand, technological development, and systemic shifts in healthcare delivery. First, the rising global burden of cardiovascular diseases, especially heart failure and coronary artery disease, is increasing the population at risk for sudden cardiac arrest and thereby expanding the need for protective interventions like WCDs. Second, growing physician awareness and guideline-based recommendations are supporting wider adoption of WCDs as interim therapy for high-risk patients not eligible for immediate ICD implantation. Third, technological improvements in device accuracy, comfort, remote monitoring, and battery performance are enhancing user experience and clinical confidence. Fourth, the healthcare industry’s pivot toward preventive care and outpatient management is creating demand for solutions that can safeguard patients outside of hospital settings. Fifth, reimbursement policy updates and increased health insurance coverage in major markets such as the United States and parts of Europe are reducing financial barriers to access. Sixth, demographic trends such as aging populations and rising incidence of lifestyle-related cardiac conditions are broadening the high-risk population segment. Seventh, increasing investment in digital health infrastructure and wearable medical technologies is fostering innovation and market entry by new players. Finally, regulatory approvals in emerging markets are opening new distribution channels and fueling geographic expansion. Together, these drivers are establishing wearable cardioverter defibrillators as a vital and rapidly growing segment within the cardiovascular device landscape.Scope of the Report
The report analyzes the Wearable Cardioverter Defibrillators market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Age Group (Pediatric Age Group, Adult Age Group, Geriatric Age Group); End-User (Home End-User, Hospitals & Cardiology Clinics End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pediatric Age Group segment, which is expected to reach US$225.7 Billion by 2030 with a CAGR of a 7.7%. The Adult Age Group segment is also set to grow at 5.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $65.3 Billion in 2024, and China, forecasted to grow at an impressive 11.3% CAGR to reach $76.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Wearable Cardioverter Defibrillators Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Wearable Cardioverter Defibrillators Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Wearable Cardioverter Defibrillators Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, AtriCure, Inc., Bard (C.R. Bard, now BD), Biotronik SE & Co. KG, Boston Scientific Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Wearable Cardioverter Defibrillators market report include:
- Abbott Laboratories
- AtriCure, Inc.
- Bard (C.R. Bard, now BD)
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cardiac Science Corporation
- CardiacSense Ltd. (adjacent wearable ECG)
- Element Science, Inc.
- Kestra Medical Technologies, Inc.
- Koninklijke Philips N.V.
- LivaNova PLC
- Medtronic plc
- Nihon Kohden Corporation
- Philips LifeVest (Philips/ZOLL)
- Schiller AG
- Sorin Group (now part of LivaNova)
- St. Jude Medical (Abbott)
- Stryker Corporation
- Welch Allyn (Hillrom/Baxter)
- ZOLL Medical Corporation (Asahi Kasei)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AtriCure, Inc.
- Bard (C.R. Bard, now BD)
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cardiac Science Corporation
- CardiacSense Ltd. (adjacent wearable ECG)
- Element Science, Inc.
- Kestra Medical Technologies, Inc.
- Koninklijke Philips N.V.
- LivaNova PLC
- Medtronic plc
- Nihon Kohden Corporation
- Philips LifeVest (Philips/ZOLL)
- Schiller AG
- Sorin Group (now part of LivaNova)
- St. Jude Medical (Abbott)
- Stryker Corporation
- Welch Allyn (Hillrom/Baxter)
- ZOLL Medical Corporation (Asahi Kasei)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 279 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
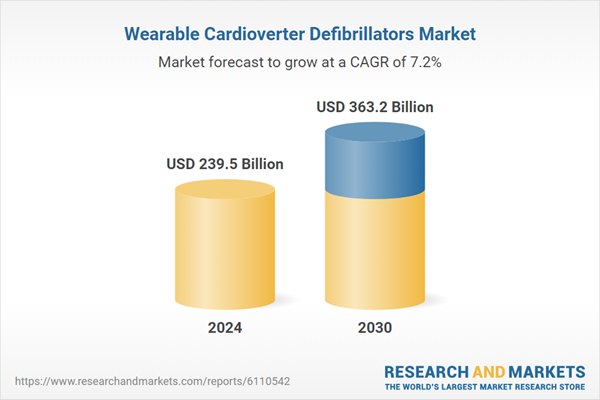
| Estimated Market Value ( USD | $ 239.5 Billion |
| Forecasted Market Value ( USD | $ 363.2 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |