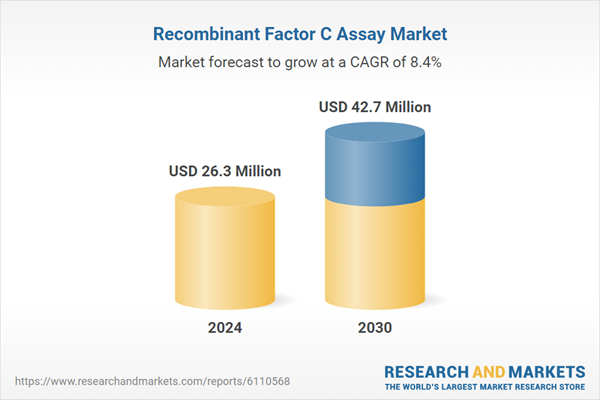
Global Recombinant Factor C Assay Market - Key Trends & Drivers Summarized
What Makes the Recombinant Factor C Assay a Disruptive Innovation in Endotoxin Detection?
The recombinant factor C (rFC) assay represents a paradigm shift in endotoxin detection technology, offering a sustainable and animal-free alternative to the traditional Limulus Amebocyte Lysate (LAL) test derived from horseshoe crab blood. rFC is engineered to replicate the critical component of the horseshoe crab’s clotting mechanism that reacts with bacterial endotoxins, making it equally sensitive while eliminating interspecies variability and ecological concerns. This innovation is driving a new industry standard in the quality control of parenteral drugs, biologics, vaccines, and medical devices where endotoxin contamination must be rigorously controlled.The regulatory momentum supporting rFC assays is growing. The United States Pharmacopeia (USP) has included rFC as an accepted compendial method, while the European Pharmacopoeia and Japanese authorities have issued comparable guidance, paving the way for global adoption. Moreover, leading pharmaceutical manufacturers are increasingly validating rFC alongside LAL in their microbial quality control processes, given its batch-to-batch consistency and reduced interference with excipients. As environmental sustainability gains traction in life sciences, the transition to rFC aligns well with corporate ESG goals and regulatory pressures to reduce dependency on endangered species and non-renewable biological materials.
How Are Industry Applications and Adoption Patterns Evolving?
The rFC assay is rapidly gaining adoption across multiple domains of the pharmaceutical manufacturing ecosystem, especially among innovators and CDMOs seeking next-generation quality assurance platforms. With increasing volumes of recombinant biologics, cell therapies, gene therapies, and monoclonal antibodies entering commercial production, endotoxin detection demands have scaled both in complexity and criticality. Unlike traditional LAL, rFC is demonstrating superior specificity in detecting Gram-negative endotoxins without false positives triggered by (1→3)-β-D-glucans, which are prevalent in certain excipients and packaging materials.Its utility in the sterile manufacturing of injectable products is especially vital, where detection limits must be low and reproducibility high. Several leading companies have adopted dual-validation protocols wherein both LAL and rFC are used in parallel during initial product development stages, gradually transitioning to full rFC workflows for ongoing release testing. This gradual transition strategy is expected to become the norm in pharma quality systems, especially as more vendors optimize assay kits for global GMP compliance. Additionally, adoption is expanding in adjacent sectors like in vitro diagnostics, medical device sterilization, and veterinary pharmaceuticals, supported by broader assay compatibility and simplified testing workflows.
What Regulatory and Supply Chain Factors Are Influencing Market Dynamics?
Regulatory standardization and supply chain resilience are key enablers of rFC assay market growth. As more health authorities endorse rFC-based methods and clarify validation pathways, pharmaceutical manufacturers are gaining the confidence to integrate rFC into primary quality control pipelines. Moreover, rFC assays offer a high degree of lot-to-lot consistency, reducing the number of invalid or inconclusive test runs-a factor that can significantly impact batch release timelines. This is particularly relevant for high-throughput GMP facilities under increasing pressure to improve time-to-market and batch clearance rates.From a supply chain standpoint, rFC assays eliminate the inherent risks associated with horseshoe crab lysate supply, which is subject to seasonal, geographic, and ecological limitations. The recombinant production process ensures consistent availability and quality, even during periods of biological or logistical disruption. Companies focusing on sterile injectables and advanced biologics are increasingly aligning with rFC assay manufacturers to ensure long-term, scalable assay supply, with some firms integrating rFC into internal sustainability targets. These supply-side advantages, combined with reduced endotoxin testing variability, position rFC as a future-proof solution for microbial safety assurance in pharmaceutical manufacturing.
Which Key Trends Are Powering Market Growth?
The growth in the recombinant factor C assay market is driven by several factors, including regulatory support, increasing biologics production, and the global push for sustainability in pharmaceutical manufacturing. A growing number of drug developers are embracing rFC for its ability to deliver accurate, reproducible endotoxin detection without the need for animal-derived reagents. As environmental conservation regulations tighten and scrutiny grows around animal-based testing, rFC’s ethical and scientific merits are gaining strategic importance in both Western and Asia-Pacific markets.Large biopharmaceutical players and vaccine manufacturers are expanding their use of rFC to cover high-throughput QC labs and in-process endotoxin screening, ensuring compliance without sacrificing ecological goals. The increasing adoption of advanced bioproduction platforms-ranging from continuous processing to single-use technologies-has also increased the need for rapid, interference-free endotoxin testing methods, further boosting rFC adoption. In addition, growing investments in pandemic preparedness, sterile injectable production, and biopharma outsourcing are expected to broaden the assay’s applicability.
Finally, with the expansion of regulatory harmonization and the scaling of recombinant assay production, rFC is poised to become the dominant method for endotoxin detection. The convergence of performance reliability, ethical considerations, and industrial scalability makes it a strong candidate to replace legacy methods and support the next chapter of microbiological safety assurance in life sciences.
Scope of the Report
The report analyzes the Recombinant Factor C Assay market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: End-User (Pharmaceutical Companies End-User, Biotechnology Companies End-User, Medical Device Companies End-User, Contract Research Organizations End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pharmaceutical Companies End-User segment, which is expected to reach US$18.2 Million by 2030 with a CAGR of a 10.3%. The Biotechnology Companies End-User segment is also set to grow at 6.0% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $7.2 Million in 2024, and China, forecasted to grow at an impressive 13.4% CAGR to reach $9.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Recombinant Factor C Assay Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Recombinant Factor C Assay Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Recombinant Factor C Assay Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam plc, Agilent Technologies Inc., Beckman Coulter Inc., Bio-Rad Laboratories Inc., Charles River Laboratories International Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Recombinant Factor C Assay market report include:
- Abcam plc
- Agilent Technologies Inc.
- Beckman Coulter Inc.
- Bio-Rad Laboratories Inc.
- Charles River Laboratories International Inc.
- DiaSorin S.p.A.
- Eurofins Scientific
- Eurogentec
- FUJIFILM Wako Pure Chemical Corporation
- Genentech Inc.
- GenScript Biotech Corporation
- Hyglos GmbH (a bioMérieux Company)
- IBA Lifesciences
- Lonza Group AG
- Merck KGaA
- PerkinElmer Inc.
- PyroGene (part of Takara Bio Inc.)
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi AppTec Co., Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam plc
- Agilent Technologies Inc.
- Beckman Coulter Inc.
- Bio-Rad Laboratories Inc.
- Charles River Laboratories International Inc.
- DiaSorin S.p.A.
- Eurofins Scientific
- Eurogentec
- FUJIFILM Wako Pure Chemical Corporation
- Genentech Inc.
- GenScript Biotech Corporation
- Hyglos GmbH (a bioMérieux Company)
- IBA Lifesciences
- Lonza Group AG
- Merck KGaA
- PerkinElmer Inc.
- PyroGene (part of Takara Bio Inc.)
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi AppTec Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 176 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 26.3 Million |
| Forecasted Market Value ( USD | $ 42.7 Million |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |