Global Nucleic Acid Based Therapeutics Market - Key Trends & Drivers Summarized
How Are Nucleic Acid Therapeutics Redefining the Future of Disease Treatment?
Nucleic acid based therapeutics are reshaping modern medicine by enabling precise manipulation of gene expression and cellular function. These therapies, which include antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), microRNAs (miRNAs), aptamers, and messenger RNAs (mRNAs), offer targeted intervention at the genetic level, bypassing conventional protein-focused approaches. Unlike traditional drugs that modulate protein pathways post-translation, nucleic acid therapeutics intervene earlier-at the transcription or translation stage-enabling profound specificity in correcting genetic dysfunctions.This transformative approach has opened doors to treating previously “undruggable” targets such as non-coding RNAs, gene fusions, and rare monogenic diseases. Therapies like nusinersen (for spinal muscular atrophy) and patisiran (for hereditary transthyretin-mediated amyloidosis) have validated the clinical viability of ASOs and siRNAs. Meanwhile, mRNA technologies-propelled to global attention by COVID-19 vaccines-are now being repurposed for oncology, autoimmune conditions, and personalized vaccines. This shift marks a transition from broad-spectrum interventions to highly individualized, gene-level medicine.
What Technological Advances Are Supporting Clinical Translation and Delivery?
The success of nucleic acid therapies hinges on delivery technologies that protect these fragile molecules and enable cell-specific uptake. Lipid nanoparticles (LNPs) have emerged as the most scalable and clinically validated carriers, particularly for mRNA and siRNA drugs. These LNPs shield therapeutic payloads from degradation, facilitate cellular entry via endocytosis, and trigger controlled release in the cytosol. Improvements in ionizable lipid design, PEGylation strategies, and targeting ligands have significantly boosted the efficacy and safety of LNP systems.Beyond LNPs, polymer-based nanoparticles, dendrimers, exosomes, and conjugated delivery using GalNAc (for liver targeting) are expanding the range of administration routes and target tissues. For example, GalNAc-conjugated siRNAs have enabled subcutaneous administration for chronic liver conditions with monthly dosing schedules. Additionally, electroporation, viral vectors (AAV, lentivirus), and novel hydrogels are being tested for localized or tissue-selective delivery in oncology and CNS disorders. Bioinformatics tools now play a pivotal role in optimizing sequence design to avoid off-target effects and enhance intracellular stability.
Simultaneously, synthetic chemistry advancements are enhancing the stability, affinity, and immunogenicity profiles of oligonucleotide sequences. Backbone modifications (e.g., phosphorothioate), sugar ring alterations (2’-O-methyl, 2’-fluoro), and locked nucleic acid (LNA) architectures have improved pharmacokinetics and minimized immune activation. These advancements are enabling more frequent dosing, longer circulation half-lives, and reduced toxicity profiles-critical factors in expanding clinical adoption and regulatory acceptance.
Which Therapeutic Areas and Companies Are Driving Market Penetration?
Rare genetic diseases, oncology, and infectious diseases are at the forefront of nucleic acid therapeutic adoption. In rare diseases, where traditional small molecules often fall short, ASOs and siRNAs have demonstrated remarkable efficacy by restoring or silencing faulty gene products. Disorders such as Duchenne muscular dystrophy, amyotrophic lateral sclerosis (ALS), and Batten disease are among the key targets under active clinical investigation. The orphan drug designation and accelerated approval pathways in the U.S. and Europe are enabling faster market entry for developers in this segment.Oncology represents a fast-emerging application area, with nucleic acid therapeutics being employed in gene silencing, neoantigen vaccine development, and immune cell reprogramming. Personalized mRNA cancer vaccines, developed in collaboration between pharma giants like Moderna and Merck, are advancing into late-stage trials for melanoma and lung cancer. Simultaneously, RNA interference platforms are being tailored to silence tumor drivers such as KRAS and STAT3. Delivery challenges in solid tumors remain a hurdle, but intratumoral and local delivery strategies are improving drug distribution and efficacy.
Leading players such as Ionis Pharmaceuticals, Alnylam, Moderna, BioNTech, and Sarepta Therapeutics dominate the landscape, leveraging deep expertise in oligonucleotide chemistry and delivery. Emerging biotechs are also innovating at the intersection of nucleic acid therapy and AI-driven drug design. Markets such as China and South Korea are accelerating homegrown development through policy support and IP harmonization, increasing regional participation in the global therapeutic pipeline.
What Is Fueling Growth in the Global Nucleic Acid Based Therapeutics Market?
The growth in the global nucleic acid based therapeutics market is driven by several factors, including rising genetic disease awareness, rapid vaccine platform development, and the growing demand for precision medicine. Increased understanding of human genomics and transcriptomics has enabled better identification of disease targets and biomarkers, expanding the pool of treatable indications through nucleic acid interventions.Clinical success stories have reduced stakeholder skepticism and accelerated investments across preclinical and clinical stages. Strategic collaborations, licensing deals, and venture capital funding are flowing into RNA-focused startups and delivery technology platforms. The success of COVID-19 mRNA vaccines served as a global validation event, prompting healthcare systems and regulators to prioritize scalable and rapid-response nucleic acid therapies for future pandemics, cancer, and emerging diseases.
Moreover, regulatory agencies have established clearer pathways and expedited review programs for these therapies, especially when targeting life-threatening conditions with high unmet needs. Reimbursement models are evolving to accommodate the high costs of personalized nucleic acid therapies, supported by outcome-based pricing and value-based contracting. With expanding manufacturing capacity, digital drug design, and increasing therapeutic diversity, nucleic acid based therapeutics are transitioning from niche biotech applications to a foundational pillar of 21st-century medicine.
Scope of the Report
The report analyzes the Nucleic Acid Based Therapeutics market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (RNA Interference & Short Interfering RNAs, Antisense Oligonucleotides, Other Products); Application (Autoimmune Disorders Application, Infectious Diseases Application, Genetic Disorders Application, Cancer Application, Other Applications) End-User (Hospitals & Clinics End-User, Academic & Research Institutes End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the RNA Interference & Short Interfering RNAs segment, which is expected to reach US$8.5 Billion by 2030 with a CAGR of a 16.3%. The Antisense Oligonucleotides segment is also set to grow at 13.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.5 Billion in 2024, and China, forecasted to grow at an impressive 20.2% CAGR to reach $2.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Nucleic Acid Based Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Nucleic Acid Based Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Nucleic Acid Based Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alnylam Pharmaceuticals, Anima Biotech, Arrowhead Pharmaceuticals, BioNTech SE, BridgeBio Pharma and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Nucleic Acid Based Therapeutics market report include:
- Alnylam Pharmaceuticals
- Anima Biotech
- Arrowhead Pharmaceuticals
- BioNTech SE
- BridgeBio Pharma
- CureVac N.V.
- Dicerna Pharmaceuticals
- Dynavax Technologies
- Gilead Sciences Inc.
- Ionis Pharmaceuticals
- Moderna Inc.
- NOXXON Pharma N.V.
- Otsuka Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Phio Pharmaceuticals Corp.
- Precision BioSciences
- ProQR Therapeutics N.V.
- Sarepta Therapeutics
- Silence Therapeutics
- Wave Life Sciences
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alnylam Pharmaceuticals
- Anima Biotech
- Arrowhead Pharmaceuticals
- BioNTech SE
- BridgeBio Pharma
- CureVac N.V.
- Dicerna Pharmaceuticals
- Dynavax Technologies
- Gilead Sciences Inc.
- Ionis Pharmaceuticals
- Moderna Inc.
- NOXXON Pharma N.V.
- Otsuka Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Phio Pharmaceuticals Corp.
- Precision BioSciences
- ProQR Therapeutics N.V.
- Sarepta Therapeutics
- Silence Therapeutics
- Wave Life Sciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 291 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
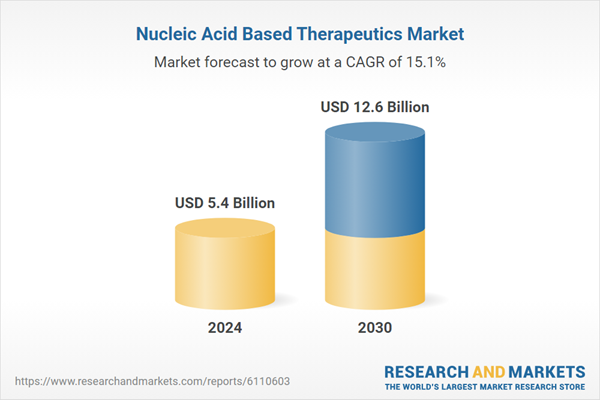
| Estimated Market Value ( USD | $ 5.4 Billion |
| Forecasted Market Value ( USD | $ 12.6 Billion |
| Compound Annual Growth Rate | 15.1% |
| Regions Covered | Global |