Global Lab Automation For In-Vitro Diagnostics Market - Key Trends & Drivers Summarized
Why Is Lab Automation Transforming the In-Vitro Diagnostics Landscape at an Unprecedented Pace?
Lab automation in the field of in-vitro diagnostics (IVD) is emerging as a pivotal driver of operational efficiency, diagnostic accuracy, and high-throughput capabilities in clinical laboratories worldwide. The surge in diagnostic demand-exacerbated by aging populations, chronic disease prevalence, and the global emphasis on personalized medicine-is compelling healthcare institutions to deploy scalable automation systems. From sample processing and reagent handling to result interpretation and data integration, automation is reducing human error and turnaround times while ensuring consistency across high-volume testing environments. Automation is no longer confined to high-end central labs; it is steadily permeating mid-size hospital labs and decentralized point-of-care (PoC) settings through modular, customizable systems.An additional factor accelerating automation is the increasing complexity and volume of diagnostic tests, including molecular diagnostics, immunoassays, and clinical chemistry panels. To keep pace, laboratories are adopting end-to-end automation solutions encompassing pre-analytical, analytical, and post-analytical stages. Instruments such as automated liquid handlers, robotic sample sorters, and integrated chemistry-analyzer systems are being adopted not only to boost throughput but also to manage biosafety risks and labor shortages. Automation is also contributing to standardization-a crucial requirement in multi-center clinical trials and national disease surveillance networks. The global push for pandemic preparedness and antimicrobial resistance monitoring is further reinforcing the importance of automated diagnostic infrastructures.
How Are Technological Innovations Reshaping Automation Workflows in IVD Labs?
Technological advancements are reshaping automation workflows in IVD by integrating robotics, AI-driven decision support, and cloud-based laboratory information systems (LIS). Robotics plays a key role in sample sorting, tube labeling, centrifugation, aliquoting, and archiving-functions that previously relied on skilled personnel. Robotic arms, conveyor tracks, and barcode scanners are now synchronized through middleware solutions, ensuring traceability and real-time analytics throughout the diagnostic pipeline. High-end automation tracks, such as Siemens' Aptio Automation and Roche’s cobas® connection modules, offer customizable configurations that allow labs to build scalable and modular systems tailored to fluctuating test volumes.Artificial intelligence and machine learning are increasingly embedded within automation platforms to support result interpretation, flagging of abnormal patterns, and predictive maintenance of equipment. In particular, AI algorithms are being trained on large datasets to reduce false positives and improve diagnostic specificity, particularly in fields like hematology and infectious disease diagnostics. Cloud-based LIS platforms are facilitating seamless data exchange across departments, institutions, and geographies, fostering collaborative diagnostics and remote monitoring. Furthermore, predictive analytics tools are helping labs forecast demand surges, optimize reagent inventory, and allocate resources dynamically-critical functions during public health emergencies.
Miniaturization and microfluidic innovations are enabling the development of compact automation systems tailored for decentralized diagnostics. These systems are particularly valuable in resource-limited settings, where infrastructure constraints previously precluded advanced diagnostics. Additionally, vendor-neutral automation interfaces are enabling interoperability among instruments from different manufacturers, making it easier for labs to adopt automation incrementally without complete overhauls. This interoperability trend is broadening adoption across emerging markets and mid-tier clinical settings, driving global market penetration.
Which End-Use Settings and Diagnostic Applications Are Accelerating Automation Adoption?
The adoption of lab automation in IVD is accelerating across various healthcare delivery models, including academic medical centers, private diagnostic labs, hospital networks, and public health agencies. Large centralized laboratories remain the most prominent adopters, utilizing full track automation systems to process tens of thousands of samples daily. However, hospital-based labs are increasingly investing in modular automation-especially for high-volume assays such as complete blood counts, thyroid function tests, and infectious disease panels. These settings benefit from improved workflow efficiency, reduced sample handling errors, and rapid response times critical for emergency and inpatient care.Molecular diagnostics is one of the fastest-growing application areas for automation, fueled by increasing demand for PCR-based and next-generation sequencing (NGS) assays. Automated nucleic acid extraction, thermal cycling, and result integration are helping labs scale their molecular testing portfolios while maintaining accuracy and compliance. Immunodiagnostics is another major segment benefiting from automation, particularly for hormone, allergy, and autoimmune panels. Meanwhile, microbiology labs are deploying automated incubators, colony pickers, and susceptibility testing systems to accelerate pathogen identification and streamline antimicrobial resistance profiling.
The rise of multi-disease test panels and syndromic testing-where multiple pathogens or biomarkers are analyzed from a single sample-is further boosting the appeal of automation in IVD. Automation platforms equipped with multiplexing capabilities enable labs to process a broader range of assays simultaneously, improving diagnostic yield and resource utilization. Public health surveillance programs, especially in infectious disease hotspots, are adopting automation to improve response times, ensure data consistency, and scale up testing capacity. As a result, the technology is extending its footprint beyond traditional urban hubs into tier-2 and tier-3 cities globally.
What Is Driving Growth in the Lab Automation for In-Vitro Diagnostics Market?
The growth in the global lab automation for in-vitro diagnostics market is driven by several factors including the rising burden of chronic and infectious diseases, demand for high-throughput diagnostics, and labor constraints in clinical laboratories. As testing volumes surge across the globe, especially in the post-pandemic era, laboratories are turning to automation as a sustainable solution to optimize operations and reduce human dependency. The increasing complexity of diagnostic tests, coupled with pressure to deliver accurate results in shorter timeframes, is fueling the transition from semi-automated to fully automated platforms.Economic drivers include the falling cost of automation systems and the availability of leasing models, which are lowering the entry barriers for mid-size labs. Government-funded programs supporting disease surveillance, population health screening, and laboratory infrastructure modernization are creating favorable procurement environments in both developed and developing regions. Regulatory encouragement for test standardization, electronic traceability, and data security is further validating the role of automation in ensuring compliance with clinical and operational mandates.
Commercial growth is also being spurred by strategic partnerships between automation providers, IVD reagent manufacturers, and LIS vendors. These collaborations are enabling the development of integrated diagnostic ecosystems that reduce interface friction and streamline end-to-end workflows. Additionally, rising awareness among healthcare administrators about return on investment (ROI) from automation-measured in terms of reduced reagent wastage, minimized reruns, and improved patient satisfaction-is accelerating budget approvals for automation upgrades. Together, these dynamics are creating a robust foundation for sustained, innovation-led growth in the lab automation for in-vitro diagnostics market.
Scope of the Report
The report analyzes the Lab Automation for In-Vitro Diagnostics market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Equipment (Automated Plate Handler, Automated Liquid Handler, Robotic Arm, Automated Storage & Retrieval System, Analyzer Equipment); End-User (Academic End-User, Laboratory End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Automated Plate Handler segment, which is expected to reach US$2.9 Billion by 2030 with a CAGR of a 4.0%. The Automated Liquid Handler segment is also set to grow at 5.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.5 Billion in 2024, and China, forecasted to grow at an impressive 7.5% CAGR to reach $1.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lab Automation for In-Vitro Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lab Automation for In-Vitro Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lab Automation for In-Vitro Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Analytik Jena, Beckman Coulter, Becton, Dickinson and Company (BD), BioMérieux and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Lab Automation for In-Vitro Diagnostics market report include:
- Abbott Laboratories
- Analytik Jena
- Beckman Coulter
- Becton, Dickinson and Company (BD)
- BioMérieux
- Bio-Rad Laboratories
- Copan Diagnostics
- Danaher Corporation
- Eppendorf AG
- Hamilton Company
- Horiba Ltd.
- LabVantage Solutions
- PerkinElmer (now Revvity)
- QIAGEN
- Roche Diagnostics
- Siemens Healthineers
- Synchron Lab Automation
- Tecan Group Ltd.
- Thermo Fisher Scientific
- Waters Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Analytik Jena
- Beckman Coulter
- Becton, Dickinson and Company (BD)
- BioMérieux
- Bio-Rad Laboratories
- Copan Diagnostics
- Danaher Corporation
- Eppendorf AG
- Hamilton Company
- Horiba Ltd.
- LabVantage Solutions
- PerkinElmer (now Revvity)
- QIAGEN
- Roche Diagnostics
- Siemens Healthineers
- Synchron Lab Automation
- Tecan Group Ltd.
- Thermo Fisher Scientific
- Waters Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 285 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
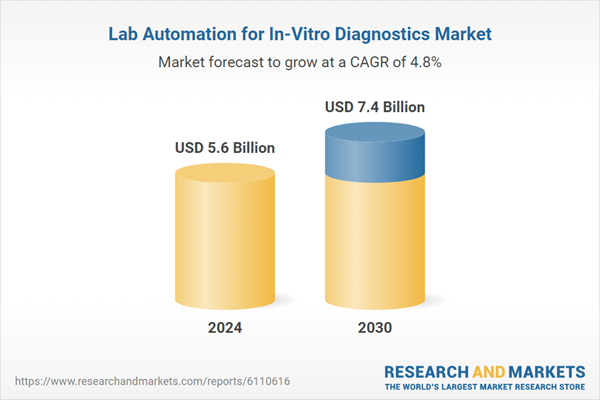
| Estimated Market Value ( USD | $ 5.6 Billion |
| Forecasted Market Value ( USD | $ 7.4 Billion |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Global |