Global Perivascular Epithelioid Cell Tumors (PEComa) Market - Key Trends & Drivers Summarized
Why Are PEComas Gaining Significance in Oncologic and Rare Tumor Management?
Perivascular epithelioid cell tumors (PEComas) represent a rare group of mesenchymal tumors characterized by the co-expression of melanocytic and smooth muscle markers. Though histologically benign in most cases, some PEComas display malignant behavior, including local invasion, recurrence, and metastasis. These tumors can arise in various organs such as the uterus, lung, gastrointestinal tract, kidneys, liver, and soft tissues, making diagnosis and treatment a challenge across disciplines.Their rarity and histopathological ambiguity have traditionally made PEComas a diagnostic dilemma. However, advances in immunohistochemistry and molecular profiling-particularly the identification of TSC1/TSC2 gene mutations and mTOR pathway activation-have significantly improved diagnostic accuracy and paved the way for targeted therapies. PEComas are now being recognized as part of a larger family of tumors associated with tuberous sclerosis complex (TSC), especially in younger individuals or those with syndromic features.
With rising awareness among oncologists and pathologists, PEComas are no longer misclassified as other smooth muscle or melanoma-like tumors. This trend is improving early identification, appropriate treatment planning, and long-term surveillance, particularly in tertiary cancer centers and sarcoma referral networks. Multidisciplinary tumor boards are now including PEComa-specific treatment algorithms that encompass surgery, molecular diagnostics, and systemic therapies.
How Are Targeted Therapies and Molecular Insights Reshaping PEComa Treatment?
The most transformative trend in PEComa treatment has been the shift toward molecularly targeted therapies, particularly those targeting the mammalian target of rapamycin (mTOR) signaling pathway. Mutations in TSC1 and TSC2, which are tumor suppressor genes, lead to constitutive activation of mTOR, making it a rational target for therapy. Drugs such as sirolimus, everolimus, and temsirolimus-originally developed as immunosuppressants or for renal cell carcinoma-are now repurposed as front-line or salvage therapies for advanced PEComas.Clinical case reports and small-scale trials have demonstrated disease stabilization and partial responses with mTOR inhibitors in patients with metastatic or unresectable PEComas. These agents are often preferred over traditional chemotherapies, which have shown limited efficacy due to the tumor’s indolent growth and low mitotic index. Ongoing trials are exploring the role of dual mTORC1/mTORC2 inhibitors and combinations with VEGF or immune checkpoint inhibitors to address resistance and expand therapeutic options.
Immunohistochemistry using HMB-45, Melan-A, SMA, and desmin, combined with next-generation sequencing (NGS), is enabling precise tumor classification and therapy guidance. Liquid biopsy and circulating tumor DNA (ctDNA) analysis may emerge as non-invasive tools for monitoring treatment response and detecting minimal residual disease. These molecular approaches are shifting the paradigm from generic oncologic treatment to personalized rare tumor care.
Which Care Settings and Regions Are Influencing PEComa Management Patterns?
Due to their rarity, PEComas are predominantly managed in academic medical centers, sarcoma specialty clinics, and cancer referral hospitals equipped with advanced diagnostics and rare tumor boards. Most cases are initially suspected during imaging for unrelated complaints or following resection of masses presumed to be fibromas, lipomas, or uterine leiomyomas. Delayed diagnosis is common, but increasing use of MRI, PET-CT, and image-guided biopsies is improving detection rates.Surgical resection remains the cornerstone of curative treatment in localized disease. However, due to potential malignant transformation, recurrence risk, and multifocality (especially in TSC patients), long-term surveillance is essential. In metastatic cases, systemic mTOR inhibitors are typically initiated at comprehensive cancer centers with access to genomic profiling. Radiotherapy is reserved for select cases, particularly where complete surgical resection is not feasible.
North America and Europe lead in terms of published case series, access to mTOR inhibitors, and diagnostic molecular tools. Asia-Pacific is seeing increasing awareness and clinical reporting, especially from Japan and South Korea. Latin America and Africa have limited case documentation, but international tumor registries and rare disease networks are helping bridge gaps in care delivery, diagnosis, and treatment standardization.
What Is Driving Growth in the Global PEComa Treatment Market?
The growth in the global PEComa market is driven by improved disease recognition, the emergence of mTOR inhibitors as targeted therapies, the expanding application of genomic diagnostics, and the development of specialized care networks for rare cancers. As more clinicians become aware of the molecular underpinnings and distinct treatment needs of PEComa, earlier diagnosis and tailored therapy are becoming standard practice.Regulatory approvals and off-label use of mTOR inhibitors have given physicians effective options for managing aggressive or unresectable PEComas. Pharmaceutical interest in repurposing cancer drugs for rare tumors is increasing, supported by orphan drug designations, fast-track pathways, and growing patient advocacy. Furthermore, improvements in rare tumor surveillance registries, NGS-based diagnostic algorithms, and multidisciplinary care models are supporting growth in both diagnosis and treatment.
As molecular medicine becomes central to oncology, PEComa care exemplifies the potential for personalized therapy in ultra-rare cancers. The continued collaboration between clinicians, researchers, and biotech developers will be crucial in expanding access, reducing misdiagnosis, and improving long-term outcomes for this enigmatic tumor family.
Scope of the Report
The report analyzes the Perivascular Epithelioid Cell Tumors (PEComa) market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (PEComa Treatment, PEComa Diagnosis); End-Use (Hospitals End-Use, Diagnostic Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the PEComa Treatment segment, which is expected to reach US$41.4 Million by 2030 with a CAGR of a 3.4%. The PEComa Diagnosis segment is also set to grow at 5.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $14.2 Million in 2024, and China, forecasted to grow at an impressive 4.3% CAGR to reach $11.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Perivascular Epithelioid Cell Tumors (PEComa) Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Perivascular Epithelioid Cell Tumors (PEComa) Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Perivascular Epithelioid Cell Tumors (PEComa) Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aadi Bioscience, AstraZeneca, Bayer, Celgene (Bristol Myers Squibb), Daiichi Sankyo and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Perivascular Epithelioid Cell Tumors (PEComa) market report include:
- Aadi Bioscience
- AstraZeneca
- Bayer
- Celgene (Bristol Myers Squibb)
- Daiichi Sankyo
- Eisai Co., Ltd.
- Exelixis
- Ipsen
- Loxo Oncology (Eli Lilly)
- Merck & Co.
- Novartis
- Pfizer
- Roche
- Sanofi
- Seagen
- Sun Pharma
- Taiho Pharmaceutical
- Takeda
- Turning Point Therapeutics
- Bristol Myers Squibb
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aadi Bioscience
- AstraZeneca
- Bayer
- Celgene (Bristol Myers Squibb)
- Daiichi Sankyo
- Eisai Co., Ltd.
- Exelixis
- Ipsen
- Loxo Oncology (Eli Lilly)
- Merck & Co.
- Novartis
- Pfizer
- Roche
- Sanofi
- Seagen
- Sun Pharma
- Taiho Pharmaceutical
- Takeda
- Turning Point Therapeutics
- Bristol Myers Squibb
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 139 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
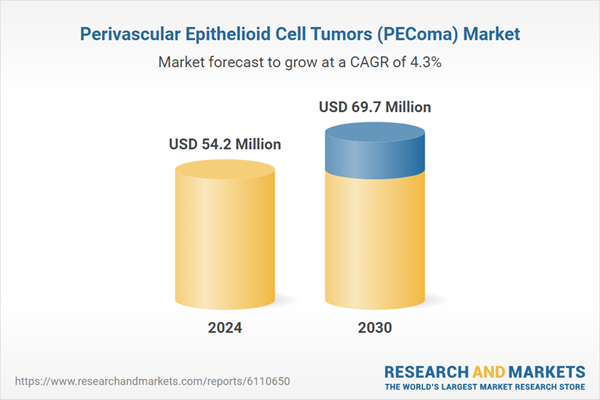
| Estimated Market Value ( USD | $ 54.2 Million |
| Forecasted Market Value ( USD | $ 69.7 Million |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | Global |