Global Pathology Devices Market - Key Trends & Drivers Summarized
How Are Pathology Devices Advancing to Meet the Demands of Precision Diagnostics?
Pathology devices are at the forefront of the medical diagnostics ecosystem, serving as the backbone of disease detection, classification, and therapeutic monitoring. The shift toward precision medicine, minimally invasive diagnostics, and early disease identification is driving innovation across a range of pathology instrumentation, including histopathology equipment, cytology systems, immunohistochemistry platforms, digital pathology scanners, and automated slide processors. These tools play an indispensable role in enabling high-throughput, reproducible, and analytically sensitive laboratory workflows.Histopathological examination remains the gold standard in cancer diagnostics and disease staging, making the demand for automated tissue processors, microtomes, embedding centers, and staining systems more critical than ever. Cytology devices, including liquid-based cytology (LBC) systems and automated slide preparers, are gaining traction in cancer screening programs, particularly for cervical and respiratory tract cancers. Immunohistochemistry (IHC), meanwhile, has seen significant adoption for biomarker detection in oncology, with antibody-based staining systems being integrated into lab automation chains for consistency and scalability.
Moreover, the advent of digital pathology is transforming slide-based diagnostics from analog to image-centric platforms. Whole-slide imaging (WSI) devices convert traditional glass slides into high-resolution digital formats, which are then analyzed via AI-powered software for cell morphology, tumor grading, and anomaly detection. This digital transition is enabling remote collaboration between pathologists, reducing inter-observer variability, and supporting telepathology for underserved and rural regions. As hospitals consolidate into integrated healthcare systems, the demand for pathology device interoperability and centralized diagnostics continues to rise.
What Technology Innovations Are Disrupting Traditional Pathology Workflows?
Digital transformation is redefining the pathology device landscape by integrating imaging, analytics, and cloud infrastructure into conventional workflows. Whole-slide scanners, capable of capturing images at 40x magnification, are increasingly equipped with high-speed autofocus, z-stacking for depth resolution, and batch scanning capabilities for large sample volumes. These devices are complemented by AI/ML algorithms that assist in quantifying tissue features, flagging potential abnormalities, and generating automated diagnostic suggestions-streamlining the pathologist’s decision-making process.Automation is another key disruptor. From robotic arms used in sample accessioning to smart staining platforms that auto-select reagents and protocols, pathology labs are minimizing manual intervention to reduce turnaround time and error rates. Modular lab automation systems now link sample tracking, barcode readers, liquid handlers, and slide cover-slippers into a seamless diagnostic pipeline, allowing laboratories to achieve faster throughput without compromising diagnostic accuracy. Integration with Laboratory Information Systems (LIS) further ensures real-time tracking, compliance, and reporting efficiency.
Additionally, the rise of companion diagnostics is influencing device development. Pharmaceutical companies and diagnostics manufacturers are collaborating to build pathology platforms that support tissue-based biomarker validation, enabling targeted therapies and personalized oncology treatments. Multiplex IHC and in situ hybridization (ISH) tools are helping laboratories detect multiple biomarkers on a single slide, increasing diagnostic yield while conserving tissue samples. These technologies are also being validated for regulatory compliance under FDA and CE marking frameworks, making them viable for clinical deployment worldwide.
Which End-Use Settings and Regional Markets Are Driving Demand for Pathology Devices?
Hospitals, diagnostic reference laboratories, academic medical centers, and cancer research institutions represent the primary end-users of pathology devices. Large hospital chains are rapidly adopting automated pathology platforms to accommodate growing biopsy volumes, shorten reporting timelines, and optimize pathology manpower. Centralized labs that process tests for multiple healthcare providers are increasingly investing in robotic and digital pathology solutions to boost efficiency and enable remote consultations.Academic institutions and cancer centers play a critical role in the early adoption of next-generation devices, particularly those involving AI interpretation and multiplex immunohistochemistry. These centers often serve as validation hubs for emerging technologies and contribute to the evidence base required for clinical and regulatory approvals. Additionally, pharmaceutical R&D and contract research organizations (CROs) are adopting digital pathology platforms for biomarker screening and drug efficacy trials-creating a growing non-clinical market for pathology devices.
Regionally, North America and Western Europe remain dominant due to advanced healthcare infrastructure, high biopsy volumes, and supportive reimbursement for pathology-based diagnostics. The U.S., in particular, is witnessing consolidation of pathology labs and strong uptake of digital pathology due to favorable CMS policies and FDA's digital imaging guidelines. Meanwhile, Asia-Pacific-led by China, India, and South Korea-is experiencing rapid growth in pathology infrastructure as cancer screening programs expand and public-private diagnostic chains scale up operations. Latin America and the Middle East are emerging markets where investments in tertiary care and cancer diagnosis are opening up new pathways for device adoption.
What Factors Are Driving the Growth of the Global Pathology Devices Market?
The growth in the global pathology devices market is driven by the increasing prevalence of chronic and oncological diseases, demand for early and accurate diagnostics, advancements in digital pathology and lab automation, and the global push toward personalized medicine. With rising cancer incidence, aging populations, and greater awareness about preventive health, there is growing pressure on diagnostic systems to deliver fast, reproducible, and actionable results-propelling the demand for advanced pathology devices.Healthcare systems worldwide are adopting centralized and digitized pathology platforms to address shortages of trained pathologists, reduce sample-to-report time, and improve diagnostic quality. In parallel, the integration of pathology with molecular diagnostics and AI analytics is enabling clinicians to extract deeper insights from tissue samples, paving the way for personalized therapy plans. These factors are particularly relevant in oncology, where treatment efficacy often depends on precise tumor classification and biomarker status.
Furthermore, strong investments from medical device firms, venture capitalists, and healthcare IT companies are accelerating innovation and commercialization. Collaborations between diagnostic labs, tech companies, and pharma players are resulting in co-developed solutions tailored for next-generation healthcare. Regulatory advancements, such as digital pathology device approvals and flexible clinical trial requirements, are further removing entry barriers. As digital and precision medicine converge, pathology devices are set to become indispensable to modern diagnostics, driving strong and sustained global market expansion.
Scope of the Report
The report analyzes the Pathology Devices market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (Clinical Chemistry Technology, Immunoassays Technology, Microbiology Technology, Molecular Diagnostics Technology, Other Technologies); Application (Drug Discovery & Development Application, Disease Diagnostics Application, Forensic Diagnostics Application, Other Applications); End-User (Pharmaceutical Companies End-User, Hospitals & Diagnostic Laboratories End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Clinical Chemistry Technology segment, which is expected to reach US$3.3 Billion by 2030 with a CAGR of a 6.0%. The Immunoassays Technology segment is also set to grow at 5.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.6 Billion in 2024, and China, forecasted to grow at an impressive 9.3% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pathology Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pathology Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pathology Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3DHISTECH, Abbott Laboratories, Agilent Technologies, Beckman Coulter (Danaher), Becton, Dickinson and Company (BD) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this Pathology Devices market report include:
- 3DHISTECH
- Abbott Laboratories
- Agilent Technologies
- Beckman Coulter (Danaher)
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories
- CellaVision
- ContextVision
- Danaher Corporation
- Epredia (3DHISTECH)
- F. Hoffmann-La Roche AG
- Hamamatsu Photonics
- Hamamatsu Photonics
- Leica Biosystems (Danaher)
- Mikroscan Technologies
- Olympus Corporation
- Ortho-Clinical Diagnostics
- PerkinElmer
- PHC Holdings / Sakura Finetek
- Philips (Digital Pathology)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3DHISTECH
- Abbott Laboratories
- Agilent Technologies
- Beckman Coulter (Danaher)
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories
- CellaVision
- ContextVision
- Danaher Corporation
- Epredia (3DHISTECH)
- F. Hoffmann-La Roche AG
- Hamamatsu Photonics
- Hamamatsu Photonics
- Leica Biosystems (Danaher)
- Mikroscan Technologies
- Olympus Corporation
- Ortho-Clinical Diagnostics
- PerkinElmer
- PHC Holdings / Sakura Finetek
- Philips (Digital Pathology)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 379 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
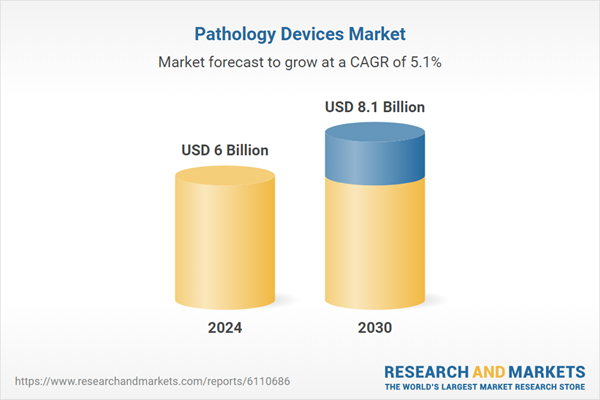
| Estimated Market Value ( USD | $ 6 Billion |
| Forecasted Market Value ( USD | $ 8.1 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |