Global Companion Diagnostic Tests in Oncology Market - Key Trends & Drivers Summarized
Why Are Companion Diagnostic Tests Revolutionizing Cancer Treatment Worldwide?
Companion diagnostic tests in oncology are fundamentally transforming how cancers are detected, classified, and treated by aligning specific therapies with individual patient profiles. These tests identify biomarkers that predict a patient’s response to a targeted therapy, making treatment more precise, effective, and personalized. Unlike conventional diagnostic approaches, companion diagnostics allow clinicians to match patients with the therapies most likely to benefit them while avoiding ineffective or potentially harmful treatments. This represents a major shift in oncology from a one-size-fits-all model to a precision medicine approach. As cancer types become increasingly segmented by genetic mutations and molecular signatures, these diagnostics are becoming indispensable in guiding therapeutic decisions. For instance, tests that detect HER2 expression in breast cancer or EGFR mutations in non-small cell lung cancer help determine eligibility for specific biologics or tyrosine kinase inhibitors. The clinical value of such testing is particularly high in cancers with well-defined genetic drivers, where outcomes are closely tied to molecular profiles. As pharmaceutical companies develop more targeted oncology drugs, the co-development of companion diagnostics is emerging as a standard strategy to gain regulatory approval and improve clinical trial success rates. The integration of these tests into early-stage cancer management is also facilitating timely intervention, improving prognosis, and enhancing patient quality of life. This confluence of clinical, economic, and technological benefits is positioning companion diagnostic tests as essential tools in the modern oncology care continuum.How Are Technological Innovations Driving Advancements in Companion Diagnostics?
Technological innovation is playing a pivotal role in enhancing the accuracy, speed, and utility of companion diagnostic tests in oncology. Advances in genomic sequencing, especially next-generation sequencing (NGS), have revolutionized how multiple cancer-related mutations can be identified from a single sample. This capability has made it feasible to develop multiplexed assays that screen for various actionable biomarkers simultaneously, providing a more comprehensive understanding of the tumor’s molecular landscape. Additionally, the emergence of liquid biopsy technology is enabling non-invasive testing through blood or other bodily fluids, eliminating the need for tissue biopsies in many cases and allowing for easier monitoring of treatment response and disease progression. Machine learning and artificial intelligence are being incorporated into companion diagnostic platforms to analyze complex genomic data, detect patterns, and generate actionable insights that support clinical decision-making. Digital pathology and imaging techniques are also contributing to improved biomarker identification by integrating visual data with molecular profiles. Furthermore, automation in laboratory workflows is increasing throughput, reducing error rates, and shortening turnaround times, making these tests more accessible to clinicians and patients. Companion diagnostics are now being integrated into digital health platforms and electronic medical records, facilitating seamless coordination between diagnostic labs, oncologists, and treatment centers. These technological advances are not only improving test sensitivity and specificity but are also expanding the range of detectable biomarkers across cancer types. As innovation accelerates, companion diagnostic tests are becoming more scalable and cost-effective, supporting their integration into routine oncology practice on a global scale.What Market and Regulatory Trends Are Shaping the Growth of Companion Diagnostics in Oncology?
The growth of the companion diagnostic market in oncology is being shaped by dynamic shifts in regulatory policy, payer reimbursement, and collaborative commercialization strategies. Regulatory agencies such as the FDA, EMA, and PMDA are increasingly recognizing the critical role of companion diagnostics in therapeutic decision-making and have established frameworks for co-approval of drugs and their corresponding diagnostic tests. These parallel approval pathways are encouraging pharmaceutical and diagnostics companies to engage in early-stage partnerships, integrating biomarker development into the drug discovery process. Reimbursement policies are evolving to support broader test accessibility, especially as health systems begin to recognize the long-term cost savings associated with precision medicine approaches that reduce treatment failure rates and adverse effects. Value-based healthcare models are emphasizing clinical utility, requiring that diagnostics demonstrate clear benefit in guiding treatment and improving outcomes. In response, diagnostic developers are investing in real-world evidence studies and health economics analyses to strengthen their value proposition to payers and providers. Intellectual property and data-sharing agreements are also becoming central to industry collaborations, as stakeholders seek to balance innovation with affordability and data interoperability. Market competition is intensifying, with both large in vitro diagnostic firms and smaller molecular diagnostics startups racing to develop biomarker panels for a growing list of targeted therapies. In parallel, governments and healthcare institutions are launching precision oncology initiatives to expand access to genomic testing and build national databases that support biomarker research. These regulatory and market forces are collectively creating a more favorable environment for the expansion of companion diagnostics, especially in high-incidence cancers and advanced therapeutic areas.What Factors Are Driving the Global Demand for Companion Diagnostic Tests in Oncology?
The growth in the companion diagnostic tests in oncology market is driven by a convergence of clinical, technological, demographic, and commercial factors that reflect the evolving landscape of cancer treatment. The increasing global cancer burden, particularly the rising incidence of solid tumors and hematologic malignancies, is creating a strong demand for diagnostics that can personalize therapy and improve treatment outcomes. As cancer care shifts toward targeted and immunotherapies, companion diagnostics are becoming essential for identifying the right patients for the right treatments, thereby enhancing efficacy and minimizing toxicity. Growing awareness among oncologists and patients about precision medicine is accelerating test adoption, particularly in developed markets with advanced healthcare infrastructure. The expanding pipeline of targeted oncology drugs, many of which require biomarker validation for regulatory approval, is fueling the co-development of companion diagnostics. Pharmaceutical companies are investing in partnerships with diagnostics firms to improve clinical trial enrollment and align drug launches with diagnostic readiness. Technological advancements such as NGS, AI-based analytics, and digital health integration are making diagnostics faster, more accurate, and easier to deploy. Government support for precision medicine initiatives and favorable reimbursement trends are further contributing to market growth. Additionally, the proliferation of molecular testing laboratories and the decentralization of diagnostic services are making companion diagnostics more accessible in emerging markets. As healthcare systems worldwide strive for more personalized, data-driven approaches to cancer care, companion diagnostic tests are emerging as indispensable tools that bridge the gap between molecular insights and therapeutic decisions. These factors collectively underscore the rapid expansion of this market and its central role in the future of oncology.Scope of the Report
The report analyzes the Companion Diagnostic Tests in Oncology market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Detection Technique (Protein Detection-Immunohistochemistry, DNA Detection-Polymerase Chain Reaction, Next-Generation Sequencing, In-Situ Hybridization, Other Detection Techniques); Biomarker (EGFR Biomarker, KRAS Biomarker, HER2 Biomarker, BRAF V600E Biomarker, Other Biomarkers); Cancer Type (Breast Cancer, Lung Cancer, Colorectal Cancer, Liver Cancer, Melanoma, Other Cancer Types); End-User (Hospitals End-User, Specialty Clinics End-User, Diagnostic Labs End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Protein Detection-Immunohistochemistry segment, which is expected to reach US$4.1 Billion by 2030 with a CAGR of a 6.9%. The DNA Detection-Polymerase Chain Reaction segment is also set to grow at 9.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.7 Billion in 2024, and China, forecasted to grow at an impressive 12.4% CAGR to reach $2.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Companion Diagnostic Tests in Oncology Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Companion Diagnostic Tests in Oncology Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Companion Diagnostic Tests in Oncology Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Agilent Technologies, Almac Group, ARUP Laboratories, bioMérieux and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Companion Diagnostic Tests in Oncology market report include:
- Abbott Laboratories
- Agilent Technologies
- Almac Group
- ARUP Laboratories
- bioMérieux
- Bio-Rad aboratories
- Caris Life Sciences
- Danaher Corporation
- Exact Sciences Corporation
- Foundation Medicine
- Guardant Health
- Illumina
- Invitae Corporation
- Myriad Genetics
- NeoGenomics Laboratories
- Qiagen
- Roche Diagnostics
- Sysmex Corporation
- Thermo Fisher Scientific
- Veracyte
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Agilent Technologies
- Almac Group
- ARUP Laboratories
- bioMérieux
- Bio-Rad aboratories
- Caris Life Sciences
- Danaher Corporation
- Exact Sciences Corporation
- Foundation Medicine
- Guardant Health
- Illumina
- Invitae Corporation
- Myriad Genetics
- NeoGenomics Laboratories
- Qiagen
- Roche Diagnostics
- Sysmex Corporation
- Thermo Fisher Scientific
- Veracyte
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 501 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
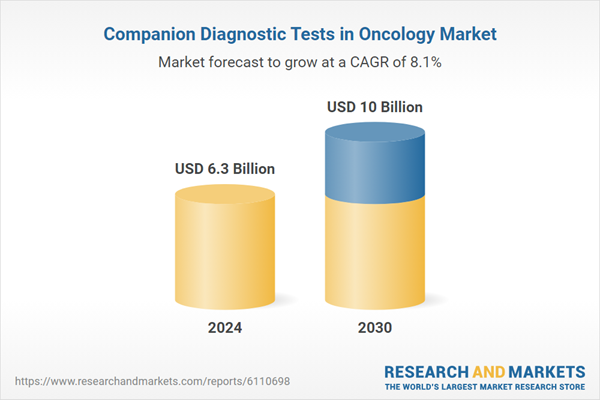
| Estimated Market Value ( USD | $ 6.3 Billion |
| Forecasted Market Value ( USD | $ 10 Billion |
| Compound Annual Growth Rate | 8.1% |
| Regions Covered | Global |