Global Sterile Vials Market - Key Trends & Drivers Summarized
Why Are Sterile Vials at the Center of Pharmaceutical & Biotech Evolution?
Sterile vials - hermetically sealed glass or plastic containers used to store injectable drugs, vaccines, and biologics - are indispensable in the pharmaceutical supply chain. Their role in ensuring drug stability, sterility, and controlled dosage delivery has grown exponentially with the rise of biologics, personalized medicine, and large-scale vaccination efforts. Unlike standard packaging containers, sterile vials must meet stringent regulatory, microbiological, and physicochemical standards to maintain product efficacy and patient safety. This is especially critical in applications such as monoclonal antibodies, gene therapies, and RNA-based drugs, where contamination or packaging-induced degradation can compromise clinical outcomes.Glass vials, particularly Type I borosilicate, dominate the market due to their chemical resistance and thermal stability. However, concerns over breakage, delamination, and leachable elements have accelerated interest in advanced polymer-based alternatives. These plastic vials offer advantages such as reduced particulate risk, lightweight design, and higher impact resistance - especially relevant for high-throughput fill-finish operations. The sterile vial market is now characterized by high-precision manufacturing, cleanroom automation, and container closure integrity testing - all of which serve the pharmaceutical industry's uncompromising demand for safety, consistency, and scalability.
How Are Innovations in Materials and Processes Redefining Sterile Vial Production?
Advancements in vial manufacturing are pushing the envelope in terms of purity, dimensional stability, and scalability. Laser-etching and camera-based inspection systems now provide 100% traceability, while integrated fill-finish lines combine washing, depyrogenation, filling, and capping under sterile, isolator-controlled conditions. This minimizes human intervention and contamination risk. New washing protocols and low-endotoxin formulations ensure that sterile vials meet strict thresholds for injectable-grade use, while advanced lyophilization-compatible designs support freeze-drying processes required for vaccines and biologics.Material innovation is equally pivotal. While glass remains dominant, the rise of cyclic olefin polymer (COP) and cyclic olefin copolymer (COC) vials presents new frontiers. These polymers offer excellent transparency, dimensional accuracy, and low extractables, making them suitable for sensitive biologics and high-purity injectable formulations. They’re also compatible with gamma and e-beam sterilization methods, broadening options for aseptic processing. Meanwhile, blow-fill-seal (BFS) technology is gaining ground in unit-dose delivery formats, allowing the sterile formation and sealing of vials in a single step - particularly useful in high-volume vaccine and ophthalmic applications.
How Are End-Use Patterns Shaping Demand for Sterile Vials?
Pharmaceutical and biotech companies are the primary consumers of sterile vials, particularly for parenteral drug delivery. The growing prevalence of chronic diseases and the surge in biologics - many of which require cold-chain preservation and aseptic packaging - have driven demand for sterile packaging solutions that offer high barrier protection and thermal resilience. Hospitals and compounding pharmacies also use sterile vials for customized admixtures, anesthetics, and diagnostic reagents.The impact of pandemic preparedness and global immunization programs cannot be overstated. Sterile vials played a pivotal role in the deployment of COVID-19 vaccines, spotlighting the need for scalable, GMP-compliant manufacturing lines and robust cold-chain packaging. As nations build long-term stockpiles and booster programs become normalized, demand for sterile vials continues to escalate - not just in developed markets but across emerging economies as well.
Veterinary medicine, contract manufacturing organizations (CMOs), and research laboratories represent other critical end users. These segments value flexibility in batch sizes, compatibility with high-potency APIs, and ease of integration into existing sterile fill-finish lines. Additionally, the rise of patient-centric dosing formats - such as self-administered injectables - is spurring interest in pre-filled sterile vial formats that simplify handling and minimize exposure risk.
What’s Driving the Growth in the Sterile Vials Market?
The growth in the sterile vials market is driven by several factors centered around advancements in vial materials, aseptic manufacturing technologies, and shifts in pharmaceutical delivery models. On the materials front, innovations in polymer vial technologies - especially those that reduce extractables and offer break resistance - are expanding usage beyond conventional glass. These new formats are particularly attractive for high-value biologics and sensitive formulations, where even trace contaminants can alter efficacy.In terms of manufacturing technology, the proliferation of robotic, isolator-based fill-finish systems ensures sterile vial production with minimal human intervention, complying with cGMP and Annex 1 guidelines. These systems offer real-time environmental monitoring, automated decontamination, and container closure integrity verification - making them vital for high-throughput, low-defect processing. Integration of serialization, RFID tagging, and camera vision systems supports full traceability and anti-counterfeiting compliance.
End-use expansion plays an equally crucial role. The shift toward complex biologics, personalized therapies, and next-gen vaccines - many of which are temperature-sensitive or require multi-dose regimens - demands packaging formats that maintain integrity under cold chain and extended storage. The rise of CDMOs and decentralized clinical trials is also increasing demand for sterile vials in smaller, more flexible batch sizes. Regulatory mandates around sterility assurance and serialization further elevate the need for precision-engineered packaging solutions.
Together, these technology-driven and industry-aligned trends are powering sustained and high-value growth in the global sterile vials market.
Scope of the Report
The report analyzes the Sterile Vials market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Sterile Empty Vial, Sterile Liquid Filled Vial, Individual Sterilized Components); Material (Glass Material, Plastic Material); Volume (Below 2 ml Volume, 2 - 5 ml Volume, 5 - 10 ml Volume, 10 - 20 ml Volume, Above 20 ml Volume); End-User (Clinical Labs End-User, Compounding Labs End-User, Biopharmaceutical Companies End-User, CMOs End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Sterile Empty Vial segment, which is expected to reach US$5.2 Billion by 2030 with a CAGR of a 5.5%. The Sterile Liquid Filled Vial segment is also set to grow at 8.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.8 Billion in 2024, and China, forecasted to grow at an impressive 10.5% CAGR to reach $2.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Sterile Vials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Sterile Vials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Sterile Vials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acme Vial and Glass Company, LLC, Adelphi Healthcare Packaging, APG Europe, Bormioli Pharma S.p.A., Corning Incorporated and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Sterile Vials market report include:
- Acme Vial and Glass Company, LLC
- Adelphi Healthcare Packaging
- APG Europe
- Bormioli Pharma S.p.A.
- Corning Incorporated
- Dalton Pharma Services
- DWK Life Sciences GmbH
- Gerresheimer AG
- Kishore Group
- Nipro Corporation
- O.Berk Company
- Pacific Vial Manufacturing, Inc.
- Piramal Glass (Piramal Enterprises Ltd.)
- Qorpak
- SCHOTT AG
- SGD Pharma
- Shandong Pharmaceutical Glass Co., Ltd.
- Stevanato Group S.p.A.
- Thermo Fisher Scientific
- West Pharmaceutical Services, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acme Vial and Glass Company, LLC
- Adelphi Healthcare Packaging
- APG Europe
- Bormioli Pharma S.p.A.
- Corning Incorporated
- Dalton Pharma Services
- DWK Life Sciences GmbH
- Gerresheimer AG
- Kishore Group
- Nipro Corporation
- O.Berk Company
- Pacific Vial Manufacturing, Inc.
- Piramal Glass (Piramal Enterprises Ltd.)
- Qorpak
- SCHOTT AG
- SGD Pharma
- Shandong Pharmaceutical Glass Co., Ltd.
- Stevanato Group S.p.A.
- Thermo Fisher Scientific
- West Pharmaceutical Services, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 481 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
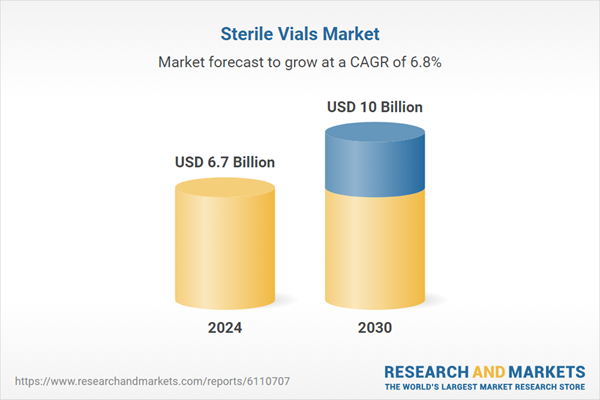
| Estimated Market Value ( USD | $ 6.7 Billion |
| Forecasted Market Value ( USD | $ 10 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |