Global Diagnostic Specialty Antibodies Market - Key Trends & Drivers Summarized
Why Are Diagnostic Specialty Antibodies Gaining Ground in Clinical Laboratories and Research Settings?
Diagnostic specialty antibodies play a pivotal role in detecting and identifying disease markers across a wide range of clinical applications. These antibodies are highly specific to unique antigens and are used to diagnose infectious diseases, autoimmune disorders, and various types of cancer. Their accuracy and reliability in immunohistochemistry, flow cytometry, and ELISA platforms have positioned them as essential reagents in laboratory medicine. Diagnostic labs and hospitals increasingly rely on specialty antibodies for precise biomarker detection and pathological analysis.In oncology, these antibodies are used for subclassifying tumor types, aiding in targeted therapy decisions. Infectious disease diagnostics also benefit from rapid antibody-based assays for early-stage pathogen detection. The rise of companion diagnostics in personalized medicine has increased demand for antibodies tailored to specific drug responses or genetic profiles. In academic research, specialty antibodies support investigations into protein expression, signaling pathways, and disease mechanisms, especially in cell and molecular biology domains.
What Technological and Process Developments Are Influencing Antibody Utility and Performance?
Monoclonal antibody production has evolved with recombinant and hybridoma technologies enabling improved purity, stability, and reproducibility. Recombinant platforms allow fine control over antibody structure and binding affinity, ensuring greater assay consistency. Labeling techniques have advanced, allowing conjugation of antibodies with fluorescent, enzyme, or radioactive markers to suit diverse detection formats.Automation in antibody screening and validation processes has reduced development time while increasing reliability. Manufacturers are incorporating multiplexing capabilities to support simultaneous detection of multiple biomarkers. Shelf life and storage stability improvements have enhanced usability in decentralized diagnostic settings. Trends also indicate a shift toward animal-free production methods and high-throughput screening systems that improve scalability and compliance with ethical standards.
Which Clinical and Industrial Settings Are Accelerating Market Demand?
Demand is rising from hospitals, pathology labs, and reference testing centers for disease-specific diagnostic panels using specialty antibodies. Clinical oncology labs utilize these tools to identify receptor status in breast, lung, and colorectal cancers. In infectious disease testing, specialty antibodies are deployed in immunoassays for tuberculosis, HIV, hepatitis, and emerging viral threats. Neurology-focused diagnostics apply antibodies to detect abnormal protein aggregates in Alzheimer’s and Parkinson’s disease.In veterinary medicine, specialty antibodies are used to identify infectious agents in livestock and companion animals. Food safety labs rely on them for detecting bacterial contaminants and allergens. Pharmaceutical firms use diagnostic antibodies in drug development to study disease models, validate targets, and monitor clinical trial biomarkers. Research institutes and CROs also utilize specialty antibodies for gene expression and tissue-specific investigations, expanding beyond routine diagnostics into translational research.
What Are the Main Drivers Behind Market Growth for Diagnostic Specialty Antibodies?
Growth in the diagnostic specialty antibodies market is driven by several factors related to precision medicine, biomarker research, and diagnostic platform integration. Expansion of personalized therapy programs in oncology and autoimmune diseases has created strong demand for companion diagnostic antibodies. Rise in infectious disease outbreaks and screening initiatives has increased the need for rapid, reliable immunoassays. Technological improvements in antibody cloning and recombinant production have enabled scale-up with improved batch consistency and customization options.Adoption of multiplex immunoassays and automated immunohistochemistry platforms in pathology labs has elevated demand for high-affinity antibodies. Development of disease-specific panels by diagnostic kit manufacturers continues to drive procurement across hospitals and research facilities. Growth in chronic disease prevalence and decentralization of diagnostics into point-of-care and home-testing formats also support higher usage of robust, pre-validated antibody reagents. Expansion of academic research in immunopathology and protein function further contributes to sustained market demand across regions.
Scope of the Report
The report analyzes the Diagnostic Specialty Antibodies market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Monoclonal Antibodies, Polyclonal Antibodies, Other Antibodies); Application (Oncology Diagnosis Application, Hepatitis Diagnosis Application, Infectious Disease Diagnosis Application, Other Applications); End-User (Diagnostic Laboratories End-User, Hospitals End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Monoclonal Antibodies segment, which is expected to reach US$20.7 Billion by 2030 with a CAGR of a 3.7%. The Polyclonal Antibodies segment is also set to grow at 2.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $7.5 Billion in 2024, and China, forecasted to grow at an impressive 6.4% CAGR to reach $6.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Diagnostic Specialty Antibodies Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Diagnostic Specialty Antibodies Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Diagnostic Specialty Antibodies Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam plc, Abnova Corporation, Agilent Technologies, Inc., Becton, Dickinson and Co., BioLegend, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Diagnostic Specialty Antibodies market report include:
- Abcam plc
- Abnova Corporation
- Agilent Technologies, Inc.
- Becton, Dickinson and Co.
- BioLegend, Inc.
- Bio-Rad Laboratories, Inc.
- Cell Signaling Technology, Inc.
- Creative Diagnostics
- Danaher Corporation (Cytiva, Leica, Beckman)
- Elabscience Biotechnology Inc.
- Enzo Life Sciences, Inc.
- GenScript Biotech Corporation
- HyTest Ltd.
- Merck KGaA (MilliporeSigma)
- Novus Biologicals, LLC
- OriGene Technologies, Inc.
- PerkinElmer, Inc. (Revvity)
- Rockland Immunochemicals Inc.
- R&D Systems (Bio-Techne)
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam plc
- Abnova Corporation
- Agilent Technologies, Inc.
- Becton, Dickinson and Co.
- BioLegend, Inc.
- Bio-Rad Laboratories, Inc.
- Cell Signaling Technology, Inc.
- Creative Diagnostics
- Danaher Corporation (Cytiva, Leica, Beckman)
- Elabscience Biotechnology Inc.
- Enzo Life Sciences, Inc.
- GenScript Biotech Corporation
- HyTest Ltd.
- Merck KGaA (MilliporeSigma)
- Novus Biologicals, LLC
- OriGene Technologies, Inc.
- PerkinElmer, Inc. (Revvity)
- Rockland Immunochemicals Inc.
- R&D Systems (Bio-Techne)
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 367 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
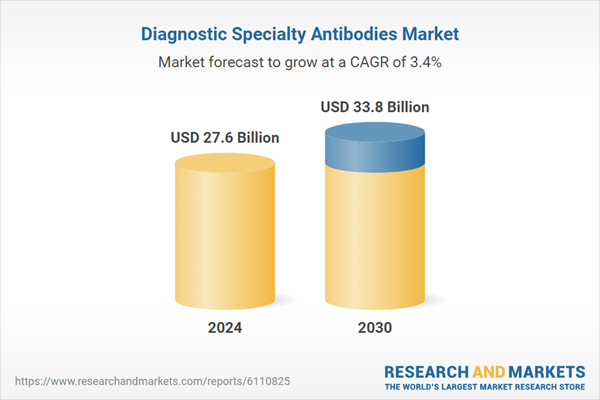
| Estimated Market Value ( USD | $ 27.6 Billion |
| Forecasted Market Value ( USD | $ 33.8 Billion |
| Compound Annual Growth Rate | 3.4% |
| Regions Covered | Global |