Global Neurofibromatosis Drugs Market - Key Trends & Drivers Summarized
Why Is Drug Development for Neurofibromatosis Entering a New Phase of Innovation?
Neurofibromatosis (NF), a group of rare genetic disorders characterized by nerve sheath tumor growth, has long posed challenges due to its diverse manifestations, chronic progression, and lack of definitive curative therapies. The three primary types-NF1, NF2, and Schwannomatosis-affect multiple systems, often leading to debilitating pain, hearing loss, disfigurement, and cognitive impairments. Until recently, treatment was largely symptomatic, focusing on surgical removal of tumors or palliative care. However, the market is now witnessing a transformative phase, with targeted drug development driven by greater molecular understanding of NF pathogenesis.The breakthrough came with the FDA approval of selumetinib, a MEK inhibitor developed by AstraZeneca, for NF1-related plexiform neurofibromas. This marked the first systemic drug for NF1, setting a precedent for targeted therapies aimed at inhibiting specific signaling pathways such as RAS/RAF/MEK/ERK, which are dysregulated in NF1 tumors. Clinical research into other kinase inhibitors, immunomodulators, and gene-silencing drugs is now expanding rapidly. This shift reflects broader trends in precision medicine and rare disease research, where regulatory incentives and orphan drug designations are accelerating development pipelines for underserved conditions like NF.
What Technological and Clinical Developments Are Accelerating Drug Pipelines?
Advances in genomic sequencing and biomarker discovery are enabling earlier and more accurate diagnosis of NF subtypes, leading to better patient stratification and targeted therapy trials. Multi-omics platforms and CRISPR screening tools are helping identify druggable mutations and tumor drivers across NF1, NF2, and Schwannomatosis. MEK inhibitors, mTOR inhibitors, and tyrosine kinase inhibitors (TKIs) are being investigated not only for tumor shrinkage but also for neurological symptom mitigation, such as attention deficits and learning disabilities in pediatric patients.Clinical trials are increasingly incorporating adaptive designs, real-world evidence, and patient-reported outcome measures (PROMs) to assess drug efficacy across diverse symptom domains. For example, trials sponsored by the Children’s Tumor Foundation and the Neurofibromatosis Therapeutic Acceleration Program (NTAP) are testing combination therapies involving selumetinib and novel biologics. Simultaneously, digital imaging and AI-driven radiological assessments are allowing for objective tumor volume tracking and better endpoint measurements in long-duration trials.
Another major trend is the focus on Schwannomatosis and NF2, both of which currently lack FDA-approved therapies. Early-stage studies targeting SMARCB1 and LZTR1 mutations are gaining momentum, while patient registries and biobank collaborations are helping researchers understand disease heterogeneity. These insights are laying the groundwork for personalized treatment algorithms and next-generation NF therapeutics.
Which Stakeholders and Regional Markets Are Influencing Drug Accessibility and Innovation?
Biopharmaceutical companies, academic research centers, and patient advocacy organizations form the cornerstone of NF drug innovation. Public-private partnerships are central to pushing forward investigational pipelines, with funding from NIH, EU Horizon programs, and rare disease venture capital firms enabling early-stage research. Orphan drug designations in the U.S. and Europe are reducing regulatory hurdles and offering market exclusivity, tax credits, and accelerated review timelines for NF therapies.The U.S. leads in both clinical development and market access, driven by robust patient advocacy groups and specialized treatment centers. Europe follows with strong research consortia and cross-border trials supported by EMA pathways for rare diseases. Asia-Pacific is witnessing emerging activity, particularly in Japan and South Korea, where rare disease initiatives are integrating NF into national clinical trial registries. In low- and middle-income regions, however, awareness and access remain limited, with NF patients often facing delays in diagnosis and lack of specialized care.
Access to therapies remains a key challenge, particularly for off-label use or investigational drugs not covered under conventional insurance. Patient assistance programs, foundation-sponsored subsidies, and compassionate use provisions are helping bridge some gaps, but broader payer frameworks are needed to ensure equitable access. As more therapies approach regulatory milestones, market players will need to address affordability, distribution, and long-term safety monitoring comprehensively.
What Is Fueling Growth in the Global Neurofibromatosis Drugs Market?
The growth in the global neurofibromatosis drugs market is driven by several factors, including increased disease awareness, landmark regulatory approvals, and deeper scientific understanding of NF biology. The success of selumetinib has validated targeted therapy approaches and catalyzed interest from pharmaceutical firms in exploring kinase pathways, immunotherapies, and even gene therapies for managing NF symptoms and tumor progression.The orphan drug framework continues to incentivize R&D, making NF an increasingly attractive domain for precision medicine investment. Cross-disciplinary research-spanning oncology, neurology, and genetics-is generating a rich pipeline of investigational drugs with diverse mechanisms of action. Furthermore, the integration of digital health tools and patient-reported data is enhancing trial efficiency and expanding the evidence base for regulatory and clinical decision-making.
As patient advocacy organizations amplify voices and demand faster access to treatments, regulatory agencies are showing greater flexibility in trial endpoints, risk-benefit analyses, and conditional approvals. The expanding global network of NF research centers, clinical trial consortia, and treatment hubs is expected to support sustained momentum. With more candidates in Phase I/II trials and a growing body of real-world data, the neurofibromatosis drugs market is positioned for accelerated expansion over the next decade.
Scope of the Report
The report analyzes the Neurofibromatosis Drugs market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Disease Type (Neurofibromatosis Type 1 Disease, Neurofibromatosis Type 2 Disease, Schwannomatosis Disease); End-User (Hospital Pharmacies End-User, Drug Stores End-User, Online Distribution Channel End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Neurofibromatosis Type 1 Disease segment, which is expected to reach US$4.2 Billion by 2030 with a CAGR of a 13.7%. The Neurofibromatosis Type 2 Disease segment is also set to grow at 10.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $833.3 Million in 2024, and China, forecasted to grow at an impressive 17.5% CAGR to reach $1.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Neurofibromatosis Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Neurofibromatosis Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Neurofibromatosis Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Amgen Inc., AstraZeneca plc, Bayer AG, Bristol Myers Squibb and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Neurofibromatosis Drugs market report include:
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- Bristol Myers Squibb
- Chugai Pharmaceutical Co.
- Deciphera Pharmaceuticals
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- GlaxoSmithKline plc
- Inhibikase Therapeutics
- Jazz Pharmaceuticals
- Kadmon Holdings, Inc.
- Loxo Oncology (Eli Lilly)
- Merck & Co., Inc.
- Novartis AG
- Recursion Pharmaceuticals
- Roche Holding AG
- SpringWorks Therapeutics
- Takeda Pharmaceutical Co.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- Bristol Myers Squibb
- Chugai Pharmaceutical Co.
- Deciphera Pharmaceuticals
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- GlaxoSmithKline plc
- Inhibikase Therapeutics
- Jazz Pharmaceuticals
- Kadmon Holdings, Inc.
- Loxo Oncology (Eli Lilly)
- Merck & Co., Inc.
- Novartis AG
- Recursion Pharmaceuticals
- Roche Holding AG
- SpringWorks Therapeutics
- Takeda Pharmaceutical Co.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 280 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
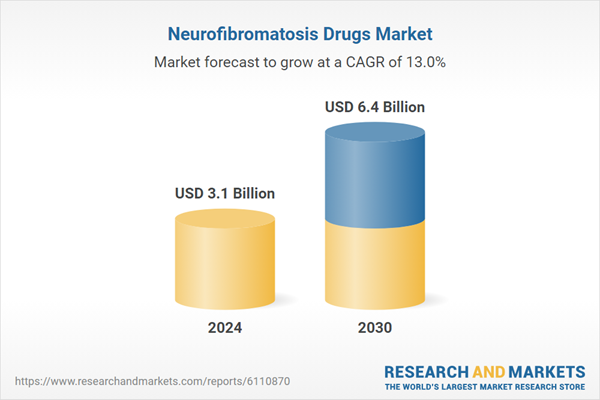
| Estimated Market Value ( USD | $ 3.1 Billion |
| Forecasted Market Value ( USD | $ 6.4 Billion |
| Compound Annual Growth Rate | 13.0% |
| Regions Covered | Global |