Global Liver Cancer Therapeutics Market - Key Trends & Drivers Summarized
Why Is the Therapeutic Landscape for Liver Cancer Rapidly Transforming?
Liver cancer, particularly hepatocellular carcinoma (HCC), represents one of the most aggressive and deadly malignancies globally, with a notably poor prognosis and rising incidence rates driven by chronic hepatitis infections, alcohol-induced cirrhosis, and increasingly, non-alcoholic steatohepatitis (NASH). The transformation of the liver cancer therapeutics landscape is being fueled by paradigm shifts from mono-therapies toward combination regimens and personalized treatment strategies. Historically dominated by sorafenib, the market has seen a wave of new approvals and pipeline candidates that are reshaping clinical practice. The shift to targeted therapies and immune checkpoint inhibitors is enabling a more tailored approach to treating liver cancer, reflecting a growing emphasis on molecular profiling, biomarker identification, and real-time disease monitoring.With regulatory bodies accelerating review timelines for breakthrough therapies, a growing number of immuno-oncology drugs such as atezolizumab and nivolumab have gained traction, especially in combination with anti-angiogenic agents like bevacizumab. This dual approach is designed to address both tumor immune evasion and vascular proliferation, offering survival benefits and enhanced progression-free outcomes. Precision medicine is becoming central to treatment decision-making, supported by companion diagnostics, circulating tumor DNA (ctDNA) assays, and genomic sequencing platforms that enable oncologists to match therapies to individual tumor signatures more effectively.
How Are Technological Advances and Treatment Modalities Reframing Patient Outcomes?
Technological advancement in interventional oncology and molecular diagnostics is critically enhancing therapeutic precision in liver cancer care. Image-guided therapies such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and selective internal radiation therapy (SIRT) continue to play pivotal roles in treating intermediate-stage HCC or when systemic therapy is contraindicated. Meanwhile, robotic-assisted liver surgery, 3D liver modeling, and augmented reality systems are improving resectability assessment and surgical outcomes. These interventions are being increasingly integrated with systemic regimens in multimodal strategies that target early-stage, unresectable, and recurrent cases.The rise of next-generation sequencing (NGS) and multi-analyte liquid biopsies has allowed oncologists to detect mutations in genes such as TP53, CTNNB1, and TERT, which have prognostic and therapeutic significance. Concurrently, clinical research is focused on identifying and targeting molecular pathways such as Wnt/β-catenin, PI3K/AKT/mTOR, and FGFR4 that drive tumor growth and resistance. Targeted kinase inhibitors beyond sorafenib and lenvatinib-such as regorafenib, cabozantinib, and ramucirumab-are being deployed in treatment-refractory settings, enhancing second- and third-line therapy options.
Gene therapy, tumor-infiltrating lymphocyte (TIL) therapies, and bispecific antibodies represent future-forward developments that are expected to further personalize liver cancer treatment. Innovations in drug delivery mechanisms-including nanoparticle carriers, liposomal systems, and liver-targeted prodrugs-are also improving bioavailability while minimizing systemic toxicity. Together, these technological and therapeutic advances are recalibrating survival expectations and establishing new standards in hepatocellular carcinoma care.
Which Markets and Segments Are Accelerating Liver Cancer Therapeutics Adoption?
The global burden of liver cancer is disproportionately concentrated in East Asia and sub-Saharan Africa, with China alone accounting for over 50% of global HCC cases. Consequently, Asia-Pacific is the largest and most dynamic market for liver cancer therapeutics, driven by high prevalence, government screening programs, and rapidly expanding oncology drug access. Japan and South Korea are also significant due to strong national cancer registries, high diagnostic awareness, and a mature biopharmaceutical industry focused on precision medicine and molecular diagnostics.North America and Europe follow closely, benefitting from strong reimbursement structures, advanced oncology infrastructure, and robust clinical trial pipelines. The U.S. market is characterized by high adoption of immune checkpoint inhibitors and molecularly guided therapies, supported by favorable FDA approvals and payer coverage for high-cost biologics. European countries, particularly Germany and the UK, are investing in early detection programs and liver disease awareness campaigns to curb late-stage presentations. The rollout of digital health platforms for remote monitoring and therapy adherence is further enhancing treatment continuity and follow-up care in these developed regions.
In contrast, Latin America, the Middle East, and parts of Southeast Asia present underpenetrated markets where late diagnosis, fragmented health systems, and limited access to modern therapeutics persist. However, international NGOs, public-private partnerships, and WHO initiatives aimed at hepatitis B and C elimination are slowly enabling earlier intervention and improved access to treatment. Pharmaceutical companies are also pursuing differential pricing, licensing agreements, and local manufacturing strategies to penetrate these cost-sensitive geographies. Additionally, with liver transplant programs expanding in emerging markets, a larger cohort of patients are now becoming eligible for curative or palliative therapeutic protocols.
What Is Fueling Growth in the Liver Cancer Therapeutics Market Globally?
The growth in the global liver cancer therapeutics market is driven by several factors, including the increasing incidence of liver cancer due to rising metabolic syndrome, viral hepatitis, and alcohol abuse, as well as the clinical shift toward combination therapies and immuno-oncology approaches. Aging populations, sedentary lifestyles, and dietary changes have led to a surge in fatty liver disease and NASH, which are major risk factors for non-viral HCC. This epidemiological shift is creating demand for tailored drug development that addresses non-cirrhotic liver cancer pathophysiology.Clinical innovation is a major growth catalyst. The expanding armamentarium of targeted therapies, checkpoint inhibitors, and angiogenesis blockers-often used in combination-has significantly broadened the treatment landscape. Regulatory fast-tracking, orphan drug designations, and expanded access programs are further accelerating the commercial availability of novel agents. Moreover, growing investments in biomarker-driven drug development and tumor microenvironment research are generating new therapeutic possibilities, including personalized vaccines, CAR-T therapies, and RNA-based therapeutics.
Economic and policy incentives are reinforcing this momentum. Governments in high-burden countries are implementing national liver cancer screening and vaccination programs, especially for hepatitis B. Pharmaceutical companies are expanding their presence in Asia-Pacific through joint ventures, clinical trial collaborations, and local R&D hubs. The increasing integration of digital health technologies-ranging from AI-based liver imaging interpretation to mobile-based patient engagement platforms-is also contributing to early diagnosis and improved patient retention in care pathways. Together, these medical, technological, and socio-political drivers are propelling robust growth in the liver cancer therapeutics market.
Scope of the Report
The report analyzes the Liver Cancer Therapeutics market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Hepatocellular Carcinoma, Cholangio Carcinoma, Hepatoblastoma, Other Types); Therapy (Targeted Therapy, Radiation Therapy, Immunotherapy, Chemotherapy).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Hepatocellular Carcinoma segment, which is expected to reach US$3.3 Billion by 2030 with a CAGR of a 15.7%. The Cholangio Carcinoma segment is also set to grow at 20.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $967.9 Million in 2024, and China, forecasted to grow at an impressive 23.0% CAGR to reach $2.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Liver Cancer Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Liver Cancer Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Liver Cancer Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Amgen Inc., AstraZeneca plc, Bayer AG, BeiGene Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Liver Cancer Therapeutics market report include:
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- BeiGene Ltd.
- Bristol-Myers Squibb Company
- CStone Pharmaceuticals
- Eisai Co., Ltd.
- Eli Lilly and Company
- Exelixis, Inc.
- F. Hoffmann-La Roche AG
- Gilead Sciences, Inc.
- H3 Biomedicine Inc.
- Innovent Biologics, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Ono Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Roche Holding AG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca plc
- Bayer AG
- BeiGene Ltd.
- Bristol-Myers Squibb Company
- CStone Pharmaceuticals
- Eisai Co., Ltd.
- Eli Lilly and Company
- Exelixis, Inc.
- F. Hoffmann-La Roche AG
- Gilead Sciences, Inc.
- H3 Biomedicine Inc.
- Innovent Biologics, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Ono Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Roche Holding AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 287 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
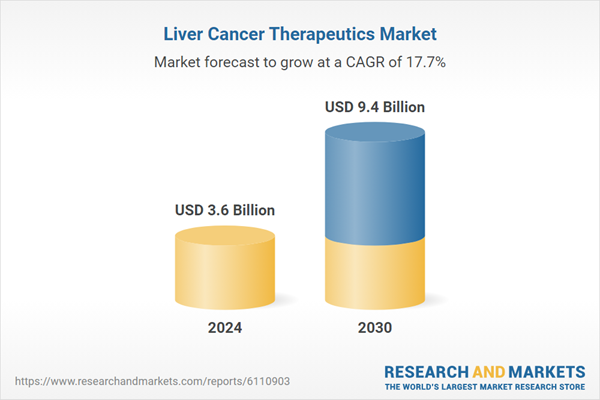
| Estimated Market Value ( USD | $ 3.6 Billion |
| Forecasted Market Value ( USD | $ 9.4 Billion |
| Compound Annual Growth Rate | 17.7% |
| Regions Covered | Global |