Global Pharmaceutical Sterile Fill-Finish Market - Key Trends & Drivers Summarized
Why Is Sterile Fill-Finish the Critical Bottleneck in Injectable Drug Manufacturing?
The sterile fill-finish process occupies a pivotal position in pharmaceutical manufacturing, as it transforms sterile drug substances into final injectable dosage forms-typically vials, prefilled syringes, ampoules, or cartridges-under aseptic conditions. While often viewed as the tail end of the production pipeline, fill-finish is among the most technically demanding and risk-sensitive stages, where product sterility, dosage accuracy, and container integrity must be preserved without compromise.The increasing global demand for biologics, vaccines, gene therapies, and complex injectables has placed unprecedented pressure on sterile fill-finish infrastructure. Biopharmaceutical products are particularly sensitive to contamination and temperature deviations, necessitating highly controlled environments with advanced isolator systems, cleanroom technologies, and validated automation workflows. Any breach during filling, capping, or lyophilization can jeopardize product efficacy and patient safety.
COVID-19 exposed the fragility of global fill-finish capacity, with limited vial availability, workforce shortages, and constrained cold chain logistics creating bottlenecks in vaccine deployment. Since then, both public and private stakeholders have ramped up investments in domestic and regional fill-finish capacity, recognizing it as a strategic imperative for supply chain resilience, regulatory compliance, and market responsiveness.
How Are Technologies Transforming Fill-Finish Efficiency and Flexibility?
Innovation across aseptic processing, robotics, and digital validation is redefining the fill-finish landscape. Barrier isolators and RABS (Restricted Access Barrier Systems) are replacing conventional cleanrooms, significantly reducing the risk of microbial contamination and minimizing operator exposure. These systems support automated, hands-free filling and capping operations, enhancing both safety and throughput.Single-use technologies are increasingly being adopted to replace traditional stainless-steel systems, offering reduced cleaning validation burden, minimized cross-contamination risk, and faster changeovers between batches. Prefilled syringe platforms and dual-chamber systems are becoming the preferred formats for self-administered biologics and emergency therapeutics, driving equipment reconfiguration and modular upgrades.
Process Analytical Technology (PAT), integrated sensors, and real-time monitoring systems are enabling continuous process verification and quality-by-design (QbD) implementation. Digital twins and AI-based predictive maintenance are being deployed to reduce downtime, simulate production scenarios, and ensure compliance across diverse product lines. Meanwhile, lyophilization innovations such as auto-loading/unloading systems, vacuum-assisted cooling, and advanced stoppering mechanisms are enhancing both freeze-drying precision and scalability.
Which Outsourcing Models and Regional Hubs Are Shaping Market Dynamics?
Contract development and manufacturing organizations (CDMOs) are playing an increasingly dominant role in the sterile fill-finish market, as pharmaceutical and biotech companies seek flexibility, speed-to-market, and capital-light scaling options. CDMOs offer end-to-end capabilities, including formulation, fill-finish, and packaging under global GMP compliance, allowing innovators to focus on clinical development while outsourcing complex manufacturing tasks.North America and Europe remain the core CDMO hubs, led by players like Catalent, Lonza, Recipharm, and Baxter BioPharma Solutions. These facilities often specialize in biologics and advanced therapies, offering high-throughput isolator lines, multi-format compatibility, and regulatory-accredited sterility assurance levels. Asia-Pacific, particularly India, China, and South Korea, is rapidly scaling capacity with cost-effective, GMP-compliant infrastructure to cater to domestic and export demand.
Public-private partnerships are also influencing regional dynamics. Initiatives like BARDA's fill-finish capacity funding in the U.S. and CEPI’s global vaccine manufacturing consortium are investing in distributed manufacturing to ensure equitable access during health emergencies. Regulatory harmonization efforts, especially among PIC/S, EMA, and FDA, are improving cross-border alignment, easing global CDMO engagement for multinational pharma companies.
What Is Driving Growth in the Global Pharmaceutical Sterile Fill-Finish Market?
The growth in the global pharmaceutical sterile fill-finish market is driven by the surge in injectable biologics and biosimilars, increasing regulatory scrutiny on sterility assurance, expansion of outsourcing to CDMOs, and adoption of automation to meet stringent GMP standards. As pipelines shift toward high-value injectables-including mRNA vaccines, monoclonal antibodies, and CAR-T therapies-fill-finish capabilities must evolve in sophistication and scale.Rising demand for personalized medicine and orphan drug formulations is spurring the need for small-batch, multi-format lines with high flexibility. The transition from traditional vials to prefilled syringes and autoinjectors for self-administration is also reshaping fill-finish infrastructure. Meanwhile, pressure to reduce time-to-market for clinical and commercial batches is fueling interest in modular, ready-to-operate fill-finish suites.
Sustainability, regulatory agility, and digital integration will further define competitive advantage. CDMOs and in-house pharma teams that invest in next-gen isolator systems, single-use platforms, and real-time process controls will be best positioned to meet the evolving demands of this critical stage in drug manufacturing. With sterile fill-finish emerging as both a quality gatekeeper and strategic bottleneck, its centrality in biopharma success is firmly established.
Scope of the Report
The report analyzes the Pharmaceutical Sterile Fill-Finish market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Vials, Prefilled Syringes, Cartridges, Other Product Types); Application (Vaccines Application, Biologics Application, Biosimilars Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Vials segment, which is expected to reach US$1.8 Billion by 2030 with a CAGR of a 2.6%. The Prefilled Syringes segment is also set to grow at 4.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.0 Billion in 2024, and China, forecasted to grow at an impressive 6.3% CAGR to reach $905.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pharmaceutical Sterile Fill-Finish Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pharmaceutical Sterile Fill-Finish Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pharmaceutical Sterile Fill-Finish Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Contract Manufacturing, Alcami Corporation, Argonaut Manufacturing Services, Catalent, Inc., Delpharm and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Pharmaceutical Sterile Fill-Finish market report include:
- AbbVie Contract Manufacturing
- Alcami Corporation
- Argonaut Manufacturing Services
- Catalent, Inc.
- Delpharm
- Fresenius Kabi
- Grand River Aseptic Manufacturing (GRAM)
- IBA Radiopharma Solutions (fill-finish)
- Intellitech Inc.
- Lonza Group
- PCI Pharma Services
- Pfizer CentreOne
- Recipharm AB
- Selkirk Pharma
- Sharp Services
- Stevanato Group
- Thermo Fisher Scientific
- Vetter Pharma-Fertigung GmbH
- West Pharmaceutical Services
- Woodstock Sterile Solutions
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Contract Manufacturing
- Alcami Corporation
- Argonaut Manufacturing Services
- Catalent, Inc.
- Delpharm
- Fresenius Kabi
- Grand River Aseptic Manufacturing (GRAM)
- IBA Radiopharma Solutions (fill-finish)
- Intellitech Inc.
- Lonza Group
- PCI Pharma Services
- Pfizer CentreOne
- Recipharm AB
- Selkirk Pharma
- Sharp Services
- Stevanato Group
- Thermo Fisher Scientific
- Vetter Pharma-Fertigung GmbH
- West Pharmaceutical Services
- Woodstock Sterile Solutions
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 291 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
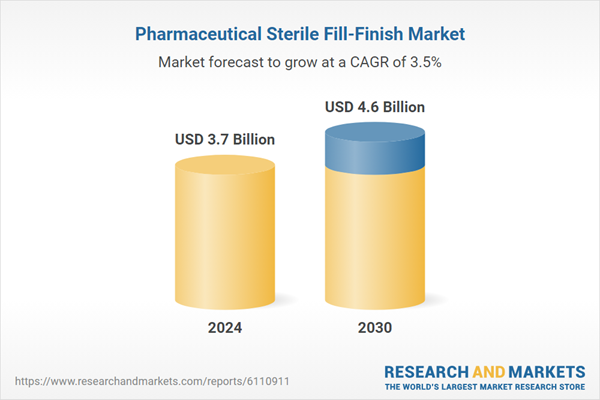
| Estimated Market Value ( USD | $ 3.7 Billion |
| Forecasted Market Value ( USD | $ 4.6 Billion |
| Compound Annual Growth Rate | 3.5% |
| Regions Covered | Global |