Global Bare-Metal Stents Market - Key Trends & Drivers Summarized
How Are Clinical Preferences and Treatment Accessibility Sustaining the Relevance of Bare-Metal Stents?
Despite the rapid advancement and adoption of drug-eluting stents, bare-metal stents continue to maintain a significant presence in interventional cardiology due to their specific clinical advantages and broader accessibility. Bare-metal stents, made primarily from stainless steel or cobalt-chromium alloys, are favored in cases where patients are unable to adhere to prolonged dual antiplatelet therapy, which is commonly required for drug-eluting stents. These stents allow for a shorter duration of post-procedure medication, making them suitable for elderly patients, individuals with bleeding risks, or those requiring urgent surgery shortly after stent placement. Additionally, bare-metal stents are often used in low-resource settings where cost is a critical consideration, as they are significantly less expensive than their drug-eluting counterparts. In many developing countries, public health systems and smaller clinics rely on these devices due to budget constraints, reinforcing their ongoing relevance. Furthermore, bare-metal stents have a long track record of safety, rapid endothelialization, and mechanical durability, which are valuable attributes in specific patient populations. As a result, interventional cardiologists continue to incorporate them into treatment plans where their unique benefits align with patient needs, reinforcing their role in a diverse range of clinical scenarios.Why Are Material Advancements and Design Improvements Enhancing Bare-Metal Stent Performance?
Technological progress in metallurgy and engineering has significantly improved the performance and clinical outcomes of bare-metal stents in recent years. Modern stents are now manufactured using high-strength alloys such as cobalt-chromium and platinum-chromium, which provide better radial strength and radiopacity while allowing for thinner struts. Thinner struts contribute to faster endothelial healing and lower risk of restenosis, without compromising mechanical integrity. Additionally, advances in laser cutting and surface finishing technologies have enhanced the precision of stent architecture, resulting in better flexibility, conformability, and ease of deployment within tortuous vessels. Surface modifications and coatings are also being developed to reduce thrombogenicity and enhance biocompatibility, even in the absence of pharmacological coatings. Computational modeling and imaging-guided design processes have enabled the optimization of cell geometry to ensure consistent expansion, reduced arterial wall stress, and minimal recoil. These innovations are helping bare-metal stents deliver improved clinical outcomes while maintaining the advantages of lower cost and simpler post-procedure management. While they may not match the long-term restenosis prevention of drug-eluting stents in all cases, these advancements are narrowing the performance gap and offering a more refined treatment option for specific patient cohorts.How Do Health System Constraints and Global Market Dynamics Influence Bare-Metal Stent Utilization?
The utilization of bare-metal stents across global healthcare systems is shaped by a complex interplay of economic, demographic, and policy factors. In many emerging economies, healthcare infrastructure is under strain due to increasing patient loads, limited budgets, and variable access to advanced medical technologies. In such environments, bare-metal stents remain a vital and cost-effective tool in the treatment of coronary artery disease. They are widely used in government-sponsored health programs, non-profit hospitals, and rural health facilities where affordability and immediate availability often outweigh long-term therapeutic optimization. Insurance coverage policies and reimbursement structures also influence stent selection, particularly in countries where public health funding does not cover high-cost interventions like drug-eluting stents. Additionally, the increasing prevalence of cardiovascular diseases in aging populations and low-income communities contributes to sustained demand for low-cost stenting solutions. In emergency procedures where rapid intervention is needed and the patient’s medication history or risk profile is unknown, clinicians may prefer bare-metal stents due to their simpler antiplatelet therapy requirements. Furthermore, ongoing clinical research and registry data collection are providing new insights into patient-specific outcomes, helping physicians make evidence-based choices that include bare-metal options in certain cases. These global market dynamics ensure that bare-metal stents remain relevant, particularly where clinical pragmatism intersects with economic realities.What Is Driving the Growth of the Global Bare-Metal Stents Market?
The growth in the global bare-metal stents market is driven by a variety of interrelated clinical, economic, and technological factors. One of the key drivers is the continued need for affordable cardiovascular interventions in low- and middle-income countries where access to premium medical devices is still limited. Bare-metal stents offer a practical and cost-effective solution for healthcare systems aiming to expand the reach of interventional cardiology. Another major growth factor is the aging global population, which is increasing the incidence of coronary artery disease and the need for less invasive, rapidly deployable treatments. Bare-metal stents are often used in high-risk patients or those requiring non-cardiac surgery shortly after angioplasty, situations where short-duration antiplatelet therapy is advantageous. The increasing awareness of cardiovascular health and the expansion of diagnostic capabilities in previously underserved regions are also contributing to a broader base of patients eligible for stenting procedures. Technological refinements in stent design, along with improvements in delivery systems and procedural efficiency, are enhancing their clinical appeal. Additionally, favorable regulatory policies in certain markets and a well-established manufacturing and distribution network continue to support steady supply and adoption. As healthcare providers strive to balance clinical efficacy with cost control, the demand for bare-metal stents is expected to persist, especially in diverse and rapidly growing markets around the world.Scope of the Report
The report analyzes the Bare-Metal Stents market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Self-Expandable Stent, Balloon Expandable Stent); Material (Stainless Steel Material, Cobalt-Chromium Alloy Material, Other Materials); End-User (Hospitals End-User, Specialized Clinics / Centers End-User, Ambulatory Surgery Centers End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Self-Expandable Stent segment, which is expected to reach US$3.6 Billion by 2030 with a CAGR of a 5.2%. The Balloon Expandable Stent segment is also set to grow at 3.0% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.1 Billion in 2024, and China, forecasted to grow at an impressive 8.3% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bare-Metal Stents Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bare-Metal Stents Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bare-Metal Stents Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Admedes GmbH, Alvimedica, AMG International GmbH, AndraTec GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Bare-Metal Stents market report include:
- Abbott Laboratories
- Admedes GmbH
- Alvimedica
- AMG International GmbH
- AndraTec GmbH
- B. Braun Melsungen AG
- Balton Sp. z o.o.
- Biosensors International Group
- BIOTRONIK SE & Co. KG
- Boston Scientific Corporation
- Cook Medical LLC
- Cordis (Cardinal Health)
- ENDOCOR GmbH
- Hexacath
- Lepu Medical Technology Co., Ltd.
- Medinol Ltd.
- Medtronic plc
- MicroPort Scientific Corporation
- Sahajanand Medical Technologies Pvt. Ltd.
- Terumo Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Admedes GmbH
- Alvimedica
- AMG International GmbH
- AndraTec GmbH
- B. Braun Melsungen AG
- Balton Sp. z o.o.
- Biosensors International Group
- BIOTRONIK SE & Co. KG
- Boston Scientific Corporation
- Cook Medical LLC
- Cordis (Cardinal Health)
- ENDOCOR GmbH
- Hexacath
- Lepu Medical Technology Co., Ltd.
- Medinol Ltd.
- Medtronic plc
- MicroPort Scientific Corporation
- Sahajanand Medical Technologies Pvt. Ltd.
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 378 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
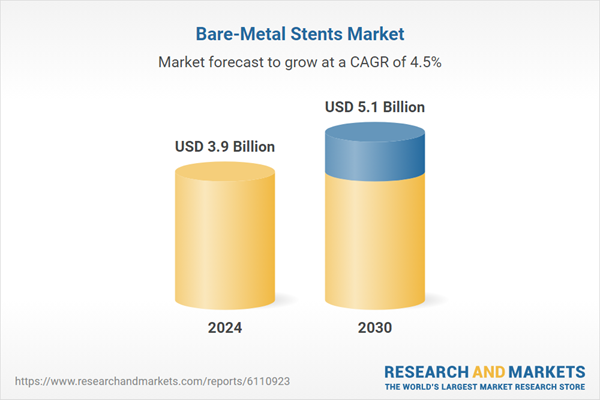
| Estimated Market Value ( USD | $ 3.9 Billion |
| Forecasted Market Value ( USD | $ 5.1 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |