Global Polypill Products Market - Key Trends & Drivers Summarized
Why Are Polypills Gaining Momentum in Cardiovascular and Chronic Disease Management?
Polypills-pharmaceutical formulations that combine multiple active ingredients into a single dosage form-are emerging as a strategic solution to address polypharmacy and improve patient adherence in the management of chronic conditions such as cardiovascular disease (CVD), hypertension, diabetes, and dyslipidemia. Typically combining a statin, a blood pressure-lowering agent, and an antiplatelet drug, polypills simplify treatment regimens for patients who require long-term management of multiple risk factors.The rationale behind polypills is grounded in clinical evidence showing that fixed-dose combinations (FDCs) can enhance medication adherence, reduce pill burden, and achieve better clinical outcomes compared to monotherapy or multiple-pill regimens. In low- and middle-income countries (LMICs), where access to healthcare is limited and follow-up inconsistent, polypills present a cost-effective intervention to reduce the global burden of non-communicable diseases (NCDs).
World Health Organization (WHO) and various cardiovascular societies have endorsed polypill-based strategies, especially for secondary prevention in patients with a history of myocardial infarction, stroke, or revascularization. With cardiovascular disease remaining the leading cause of mortality globally, polypills offer a population-level tool to standardize treatment protocols and improve public health outcomes.
What Innovations Are Enhancing the Therapeutic Utility of Polypill Products?
Pharmaceutical companies and research institutions are focusing on designing polypill formulations that are both clinically effective and patient-friendly. Innovations include enteric-coated, bilayered, or modified-release dosage forms that allow for sequential or time-delayed release of active ingredients, minimizing pharmacokinetic interactions and side effects. These delivery platforms enhance bioavailability while preserving the pharmacodynamic integrity of individual agents.Novel polypills are being designed to target multiple pathways beyond cardiovascular risk, incorporating drugs for diabetes (e.g., metformin), hyperuricemia, or mental health conditions such as depression. Such multidimensional formulations are particularly beneficial for elderly populations or those with multimorbidity, where polypharmacy increases the risk of non-adherence, drug interactions, and adverse events.
Digitally-enabled polypill strategies are also emerging, integrating smart packaging, adherence trackers, and telehealth solutions to monitor compliance and personalize dosing. The use of real-world evidence (RWE) and pragmatic clinical trials is expanding the knowledge base for polypill efficacy, enabling regulators and payers to assess long-term benefits and cost-effectiveness in routine care.
Which Patient Populations and Markets Are Accelerating Adoption of Polypill Therapies?
Secondary prevention of cardiovascular events remains the largest and most well-established use case for polypills. Patients with prior cardiovascular events or those with a high risk of recurrence benefit most from a simplified, once-daily pill that covers lipid-lowering, antihypertensive, and antiplatelet actions. Primary prevention among at-risk populations-such as individuals with metabolic syndrome, diabetes, or family history of heart disease-is also gaining traction, especially in resource-limited settings.Emerging economies, particularly in Latin America, South Asia, and Sub-Saharan Africa, are witnessing the most aggressive deployment of polypills through national health programs and public-private partnerships. India, for instance, has launched low-cost polypill initiatives under its Ayushman Bharat scheme to combat the growing NCD burden. In high-income countries, adoption is slower but increasing in specific cohorts such as geriatric patients, rural populations, or managed care settings.
Hospitals, government health departments, NGOs, and insurance providers are key stakeholders in driving adoption. As evidence from implementation science and outcome studies accumulates, polypills are being integrated into clinical practice guidelines, electronic medical records, and mobile health platforms that promote continuity of care and medication adherence.
What Is Driving Growth in the Global Polypill Products Market?
The growth in the global polypill products market is driven by rising prevalence of chronic non-communicable diseases, increasing focus on treatment adherence, and policy shifts favoring preventive health strategies. As healthcare systems grapple with aging populations, multimorbidity, and limited provider-patient contact, polypills provide a pragmatic solution to streamline therapy and reduce disease burden.Favorable clinical trial outcomes from studies such as PolyIran, TIPS-3, and UMPIRE have validated the efficacy of polypill-based regimens in reducing cardiovascular events and improving treatment adherence across diverse populations. Regulatory approvals and WHO endorsements are further enhancing physician confidence and payer willingness to reimburse these products.
Cost savings from fewer hospitalizations, reduced medication wastage, and improved long-term outcomes are attracting public health systems to incorporate polypills into essential medicine lists. Advances in pharmaceutical formulation, coupled with growing demand for patient-centric healthcare, are transforming polypills from a niche innovation to a central component of chronic disease management in both developed and developing healthcare markets.
Scope of the Report
The report analyzes the Polypill Products market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Single Parameter Polypill, Multi-parameter Polypill); Application (Cardiovascular Application, Diabetes Mellitus Application, Other Applications); Distribution Channel (Online Distribution Channel, Hospital Pharmacies, Retail Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Single Parameter Polypill segment, which is expected to reach US$23.9 Billion by 2030 with a CAGR of a 1.9%. The Multi-parameter Polypill segment is also set to grow at 0.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $8.7 Billion in 2024, and China, forecasted to grow at an impressive 3.0% CAGR to reach $6.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Polypill Products Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Polypill Products Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Polypill Products Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ajanta Pharma Ltd., Apotex Inc., Aurobindo Pharma Ltd., Bayer AG, Cipla Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Polypill Products market report include:
- Ajanta Pharma Ltd.
- Apotex Inc.
- Aurobindo Pharma Ltd.
- Bayer AG
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Gilead Sciences, Inc.
- GlaxoSmithKline plc (GSK)
- Hikma Pharmaceuticals PLC
- Intas Pharmaceuticals Ltd.
- Lupin Limited
- Mylan N.V. (part of Viatris)
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Servier Laboratories
- Strides Pharma Science Ltd.
- Sun Pharmaceutical Industries
- Teva Pharmaceutical Industries
- Torrent Pharmaceuticals Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ajanta Pharma Ltd.
- Apotex Inc.
- Aurobindo Pharma Ltd.
- Bayer AG
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Gilead Sciences, Inc.
- GlaxoSmithKline plc (GSK)
- Hikma Pharmaceuticals PLC
- Intas Pharmaceuticals Ltd.
- Lupin Limited
- Mylan N.V. (part of Viatris)
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Servier Laboratories
- Strides Pharma Science Ltd.
- Sun Pharmaceutical Industries
- Teva Pharmaceutical Industries
- Torrent Pharmaceuticals Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 378 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
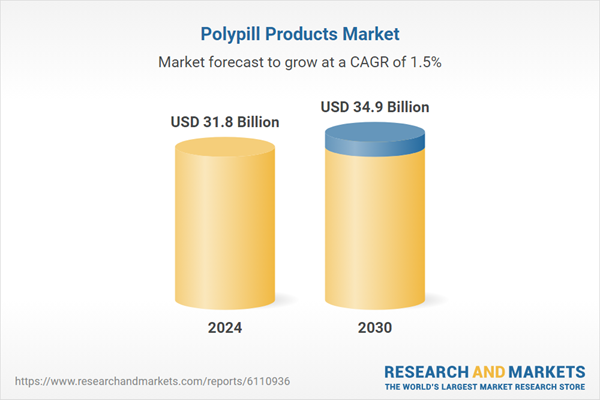
| Estimated Market Value ( USD | $ 31.8 Billion |
| Forecasted Market Value ( USD | $ 34.9 Billion |
| Compound Annual Growth Rate | 1.5% |
| Regions Covered | Global |