Global HER2 Breast Cancer Tests Market - Key Trends & Drivers Summarized
Why Are HER2 Breast Cancer Tests Essential in Modern Oncology?
HER2 (Human Epidermal Growth Factor Receptor 2) breast cancer tests are diagnostic procedures used to determine the overexpression or amplification of the HER2 gene in breast cancer tissue. These tests are critical in guiding treatment decisions, as HER2-positive cancers typically grow faster and respond differently to targeted therapies. Accurate identification allows for the selection of therapies such as trastuzumab or pertuzumab, which specifically inhibit HER2 activity and improve survival outcomes.Testing is typically performed using immunohistochemistry (IHC) or in situ hybridization (ISH) techniques on biopsy or surgical tissue samples. In recent years, advanced assays, digital pathology solutions, and companion diagnostics have emerged to improve diagnostic precision. HER2 testing is now a routine part of breast cancer diagnosis in oncology centers, contributing to the growing demand for high-quality and reliable diagnostic platforms.
What Diagnostic Innovations Are Influencing Test Accuracy and Adoption?
Technological advancements have improved HER2 test sensitivity, reproducibility, and interpretability. IHC tests have evolved with enhanced antibodies, automated staining systems, and standardized scoring algorithms. Dual-probe ISH tests provide confirmation in ambiguous cases, helping resolve borderline HER2 expressions. Fluorescence ISH (FISH) and chromogenic ISH (CISH) remain widely used for confirming gene amplification at the cellular level.Emerging platforms are integrating digital imaging, AI-assisted pathology, and multiplex testing capabilities to enhance diagnostic workflows. Some HER2 assays are now being developed as part of broader genomic profiling tests that assess multiple biomarkers simultaneously. Companion diagnostic tests co-developed with targeted drugs are also gaining traction, especially as HER2-targeted treatments expand into other tumor types like gastric and colorectal cancers.
Where Is Testing Volume Increasing, and Which Institutions Are Driving Uptake?
Testing volumes are rising globally due to increasing breast cancer incidence and the growing adoption of personalized medicine in oncology. Hospitals, cancer specialty centers, and reference laboratories are key testing sites. In high-income countries, HER2 testing is a standard part of breast cancer workups, while in middle-income regions, uptake is increasing with improvements in pathology infrastructure and treatment access.North America and Europe are mature markets with well-established diagnostic guidelines and reimbursement systems. Asia Pacific is witnessing rapid growth due to rising breast cancer awareness, expanding access to targeted therapies, and government initiatives supporting oncology diagnostics. The development of public-private partnerships and national cancer screening programs is further accelerating testing penetration.
What Is Driving Growth in the HER2 Breast Cancer Tests Market?
Growth in the HER2 breast cancer tests market is driven by several factors including rising global breast cancer incidence, increased use of HER2-targeted therapies, and ongoing improvements in diagnostic precision. Advances in staining reagents, automation systems, and AI-powered pathology tools are making HER2 testing more accurate and scalable.End-use expansion in oncology hospitals, pathology labs, and companion diagnostics for new HER2-targeted drugs is boosting market relevance. Regulatory approvals for HER2 testing in tumor types beyond breast cancer and increased adoption of multiplex biomarker panels are broadening application scope. As precision medicine becomes central to oncology care, demand for reliable, high-throughput HER2 testing solutions is expected to grow steadily.
Scope of the Report
The report analyzes the HER2 Breast Cancer Tests market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Test Type (Immunohistochemistry Test, Fluorescence / Chromogenic In-Situ Hybridization Test); End-User (Hospitals End-User, Diagnostic Laboratories End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Immunohistochemistry Test segment, which is expected to reach US$331.3 Million by 2030 with a CAGR of a 4.0%. The Fluorescence / Chromogenic In-Situ Hybridization Test segment is also set to grow at 6.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $107.4 Million in 2024, and China, forecasted to grow at an impressive 7.8% CAGR to reach $104.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global HER2 Breast Cancer Tests Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global HER2 Breast Cancer Tests Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global HER2 Breast Cancer Tests Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Abnova Corporation, Agilent Technologies, Bio-Genex Laboratories, Beckman Coulter (Danaher) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 37 companies featured in this HER2 Breast Cancer Tests market report include:
- Abbott Laboratories
- Abnova Corporation
- Agilent Technologies
- Bio-Genex Laboratories
- Beckman Coulter (Danaher)
- Becton, Dickinson and Company (BD)
- EKF Diagnostics
- Hologic, Inc.
- Leica Biosystems (Danaher)
- Meridian Bioscience
- Olympus Corporation
- Oxford Gene Technology (Sysmex)
- QIAGEN N.V.
- REVEAL Genomics S.L.
- Roche Diagnostics (F. Hoffmann-La Roche)
- Thermo Fisher Scientific
- Veracyte, Inc.
- NeoGenomics Laboratories, Inc.
- Empire Genomics / Biocare Medical
- Applied Spectral Imaging
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Abnova Corporation
- Agilent Technologies
- Bio-Genex Laboratories
- Beckman Coulter (Danaher)
- Becton, Dickinson and Company (BD)
- EKF Diagnostics
- Hologic, Inc.
- Leica Biosystems (Danaher)
- Meridian Bioscience
- Olympus Corporation
- Oxford Gene Technology (Sysmex)
- QIAGEN N.V.
- REVEAL Genomics S.L.
- Roche Diagnostics (F. Hoffmann-La Roche)
- Thermo Fisher Scientific
- Veracyte, Inc.
- NeoGenomics Laboratories, Inc.
- Empire Genomics / Biocare Medical
- Applied Spectral Imaging
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 268 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
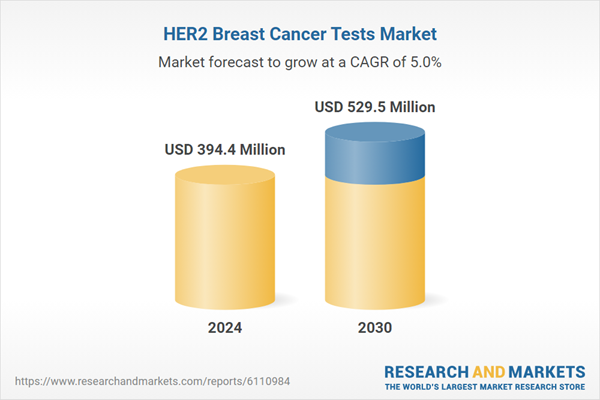
| Estimated Market Value ( USD | $ 394.4 Million |
| Forecasted Market Value ( USD | $ 529.5 Million |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |