Global Rituximab Market - Key Trends & Drivers Summarized
Why Is Rituximab Still Holding Strong in the Evolving Landscape of Targeted Biologics?
Rituximab, a chimeric monoclonal antibody targeting CD20-positive B cells, continues to be a cornerstone in the treatment of hematological malignancies and autoimmune disorders. Even two decades after its introduction, it maintains a solid presence across indications such as non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL), rheumatoid arthritis (RA), and granulomatosis with polyangiitis. Despite the emergence of newer biologics and small-molecule alternatives, rituximab remains indispensable due to its proven efficacy, well-documented safety profile, and inclusion in first-line therapy guidelines globally.The drug's broad usage in both oncology and immunology offers it a dual-market advantage. Its mechanism-targeting CD20 to mediate B-cell lysis through antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC)-has remained clinically relevant, especially in patients exhibiting B-cell dysregulation. Moreover, clinical trials continue to reinforce its use in off-label conditions like systemic lupus erythematosus (SLE) and idiopathic thrombocytopenic purpura (ITP), further expanding its therapeutic footprint. Despite competition, rituximab’s enduring clinical utility is attributed to its unique pharmacodynamics and manageable toxicity, making it a reliable tool in combination regimens.
How Are Biosimilars and Subcutaneous Formulations Reshaping Market Dynamics?
The global rituximab market is undergoing a structural shift due to the advent of biosimilars. Regulatory approvals across Europe, India, Japan, and the U.S. have facilitated the entry of cost-effective alternatives such as Truxima, Ruxience, and Reditux, which have quickly gained traction in both public health systems and private oncology clinics. These biosimilars are helping to democratize access to biologic therapies in low- and middle-income countries and are compelling originator companies to revisit pricing and distribution strategies.Another pivotal trend is the transition from intravenous (IV) to subcutaneous (SC) formulations, aimed at improving patient convenience, reducing hospital chair time, and lowering administration costs. Subcutaneous rituximab, combining the antibody with hyaluronidase for faster absorption, has gained clinical approval and patient preference in several regions. This shift is fostering home-based administration models, particularly in high-income countries with overburdened outpatient infusion centers. Meanwhile, combination products that include rituximab with other agents in pre-packaged kits are being explored to streamline chemotherapy cycles and enhance compliance in complex treatment regimens.
Which Indications and Regional Markets Are Driving Demand Diversification?
While B-cell lymphomas remain the primary domain for rituximab, its usage in autoimmune diseases is gaining pace. In rheumatoid arthritis, it serves as a second-line option after TNF inhibitors, especially in seropositive patients. It is also being increasingly considered for vasculitis syndromes and multiple sclerosis, particularly in patients with intolerance or non-responsiveness to other disease-modifying agents. This shift is encouraging multi-specialty adoption, extending rituximab use beyond oncology to rheumatology and neurology practices.Regionally, Europe has led in biosimilar adoption, with national tenders and public insurance schemes accelerating substitution from reference rituximab. India and Brazil have seen significant volume growth driven by domestic manufacturing and state procurement programs. Meanwhile, North America-especially the U.S.-still sees dominant use of branded Rituxan, although biosimilars are rapidly encroaching into hospital formularies. In Africa and Southeast Asia, demand is tied to global access programs and WHO prequalification, which are expanding rituximab availability for lymphomas under resource-limited settings. This layered growth across markets with varied pricing, regulatory, and clinical practices underscores the drug's global relevance.
What Strategic Factors Are Sustaining Market Expansion and Therapy Optimization?
The growth in the rituximab market is driven by several factors, including increasing prevalence of lymphoproliferative disorders, improved diagnosis of autoimmune diseases, expanding biosimilar penetration, and formulation innovation. The steady inclusion of rituximab in evolving treatment guidelines for both malignancies and immune-mediated conditions ensures sustained clinician trust and widespread usage. Moreover, growing awareness of early B-cell involvement in multiple inflammatory pathways is encouraging its repositioning in newer autoimmune indications.Pharmaceutical strategies are focusing on lifecycle extension through combination trials, real-world data studies, and biosimilar optimization. Several manufacturers are investing in SC formulation improvements, fixed-dose combinations, and AI-enabled pharmacovigilance to enhance safety monitoring in long-term use. Meanwhile, governments and NGOs are funding rituximab procurement under cancer care access programs, especially in underserved markets. These combined efforts-across R&D, policy, and access-are sustaining rituximab’s status as a mainstay therapy while supporting its evolution in the face of competitive and economic pressures.
Scope of the Report
The report analyzes the Rituximab market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Administration Route (Subcutaneous Administration, Intravenous Administration, Parenteral Administration); Distribution Channel (Hospitals Pharmacy, Online Pharmacy, Other Distribution Channels); Application (Non-Hodgkin Lymphoma Application, Chronic Lymphocytic Leukemia Application, Rheumatoid Arthritis Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Subcutaneous Administration segment, which is expected to reach US$6.4 Billion by 2030 with a CAGR of a 15.3%. The Intravenous Administration segment is also set to grow at 13.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.3 Billion in 2024, and China, forecasted to grow at an impressive 19.5% CAGR to reach $2.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Rituximab Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Rituximab Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Rituximab Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amgen Inc., Amneal Pharmaceuticals, Apotex Inc., Biocon Limited, Bio-Thera Solutions and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Rituximab market report include:
- Amgen Inc.
- Amneal Pharmaceuticals
- Apotex Inc.
- Biocon Limited
- Bio-Thera Solutions
- Celltrion Healthcare Co. Ltd.
- Dr. Reddy’s Laboratories
- Fresenius Kabi
- Glenmark Pharmaceuticals
- Hikma Pharmaceuticals
- Intas Pharmaceuticals Ltd.
- Mabion S.A.
- Mylan N.V. (Viatris)
- Novartis AG (Sandoz)
- Pfizer Inc.
- Reliance Life Sciences
- Roche Holding AG
- Samsung Bioepis
- Teva Pharmaceutical Industries
- Zydus Lifesciences Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amgen Inc.
- Amneal Pharmaceuticals
- Apotex Inc.
- Biocon Limited
- Bio-Thera Solutions
- Celltrion Healthcare Co. Ltd.
- Dr. Reddy’s Laboratories
- Fresenius Kabi
- Glenmark Pharmaceuticals
- Hikma Pharmaceuticals
- Intas Pharmaceuticals Ltd.
- Mabion S.A.
- Mylan N.V. (Viatris)
- Novartis AG (Sandoz)
- Pfizer Inc.
- Reliance Life Sciences
- Roche Holding AG
- Samsung Bioepis
- Teva Pharmaceutical Industries
- Zydus Lifesciences Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 378 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
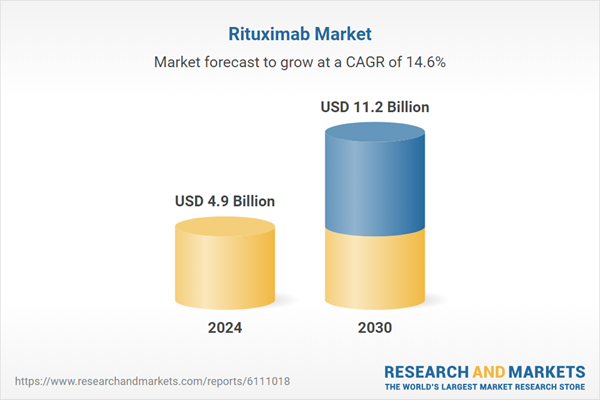
| Estimated Market Value ( USD | $ 4.9 Billion |
| Forecasted Market Value ( USD | $ 11.2 Billion |
| Compound Annual Growth Rate | 14.6% |
| Regions Covered | Global |