Global NICU Catheters Market - Key Trends & Drivers Summarized
Why Are Catheter Technologies So Critical in Neonatal Intensive Care Units?
Catheters are indispensable in Neonatal Intensive Care Units (NICUs), where premature and critically ill newborns often require precise, continuous access to vascular routes for nutrition, medication, and monitoring. Unlike adult patients, neonates have extremely delicate veins and skin, making catheterization technically demanding and clinically sensitive. As a result, the NICU catheters market has evolved to support the unique needs of neonates, offering catheters that are softer, smaller in diameter, and more biocompatible than those used in other populations. The trend toward minimally invasive neonatal care has catalyzed the development of catheters that ensure reduced trauma, infection risk, and long-term vascular complications.Central venous catheters (CVCs), peripherally inserted central catheters (PICCs), umbilical vein catheters (UVCs), and umbilical artery catheters (UACs) are commonly deployed in NICU settings. These devices provide essential access routes for administering parenteral nutrition, surfactants, vasopressors, and antibiotics, or for measuring arterial blood gases and hemodynamic status. Advanced versions come equipped with antimicrobial coatings, pressure-resistant lumens, and ultrasound-visibility features to enhance placement accuracy and patient safety. As neonatal care standards rise globally, the need for technologically advanced, risk-mitigated catheter systems is growing proportionately.
What Design Innovations Are Addressing the Challenges of Neonatal Vascular Access?
Given the anatomical and physiological fragility of neonates, NICU catheter technology has seen significant design upgrades in terms of material flexibility, insertion techniques, and safety mechanisms. Manufacturers are increasingly using biocompatible materials such as polyurethane and silicone, known for their reduced thrombogenicity and lower incidence of vascular irritation. Some catheters are embedded with heparin coatings or antimicrobial agents like silver sulfadiazine or chlorhexidine to reduce infection rates in immunocompromised infants.The use of integrated guidewire systems and pre-assembled kits has simplified the insertion process, reducing procedure time and need for skilled personnel. Ultrasound-guided catheterization is becoming standard practice, particularly for central and PICC lines, ensuring real-time vein visualization and reducing misplacement. Pressure transducer-enabled arterial catheters offer continuous monitoring without the need for repeated blood draws-especially critical in preterm neonates where blood volume is limited.
Safety-lock connectors, color-coded lumens, and radiopaque tips are further innovations enhancing usability and reducing errors. For long-term neonatal care, catheter stabilization devices and sutureless securement systems help prevent displacement and catheter-related bloodstream infections (CRBSIs). These incremental design refinements are collectively improving survival outcomes and reducing NICU stays, thus lowering the overall cost burden on healthcare systems.
Which Healthcare Systems and Regions Are Driving NICU Catheter Utilization?
NICU catheter adoption is highest in advanced healthcare markets with strong neonatal care infrastructure such as the United States, Canada, Germany, Japan, and the Nordic countries. In these regions, adherence to clinical guidelines such as those from the CDC, INS (Infusion Nurses Society), and Neonatal Resuscitation Program (NRP) is pushing hospitals toward premium, safety-enhanced catheter systems. The presence of well-trained neonatal intensivists and support staff also facilitates the integration of advanced catheter technologies into routine clinical practice.In emerging economies like India, Brazil, China, and South Africa, the rise in institutional births and government investments in neonatal mortality reduction are driving adoption. Public-private partnerships and donor-funded programs are helping equip district-level hospitals with modern NICU infrastructure, including vascular access kits and trained personnel. NGOs and health agencies working under the Sustainable Development Goals (SDGs) framework are also playing a critical role in spreading awareness about safe neonatal catheterization.
Private hospitals and specialty neonatal centers represent high-opportunity zones in Asia-Pacific and Latin America. These institutions are adopting disposable catheter kits and investing in vascular access devices that reduce procedural complications and align with global best practices. Furthermore, academic medical centers are increasingly conducting research on catheter-associated risks and innovations, creating feedback loops that refine product development and clinical training.
What Is Fueling Growth in the Global NICU Catheters Market?
The growth in the global NICU catheters market is driven by several factors, including the rising prevalence of preterm births, increasing investment in neonatal healthcare infrastructure, and technological advancements that enhance safety and clinical efficiency. According to WHO estimates, over 15 million babies are born prematurely each year, many of whom require intensive vascular access for survival. This demand has created a robust market for catheters tailored to neonatal anatomy and physiology.Regulatory support and clinical guidelines emphasizing infection control, catheter standardization, and quality improvement are propelling hospitals to upgrade legacy systems. Increasing reimbursement for neonatal intensive care, particularly in developed countries, further supports the adoption of premium catheter solutions. Additionally, the demand for disposable and single-use devices in NICUs has surged due to heightened awareness around hospital-acquired infections and cross-contamination, especially in the post-COVID healthcare landscape.
Innovations in catheter insertion training, such as simulation-based modules and augmented reality (AR) tools, are equipping caregivers with the skills necessary for high-precision catheterization. Vendors are responding with user-friendly, ergonomic designs and value-added features that support both safety and efficiency. As neonatal care becomes more personalized and technologically integrated, the NICU catheters market is poised for sustained expansion across geographies.
Scope of the Report
The report analyzes the NICU Catheters market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Peripherally Inserted Central Catheters, Central Venous Catheters, Umbilical Venous Catheters, Other Types); End-User (Hospitals End-User, Specialty Clinics End-User, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Peripherally Inserted Central Catheters segment, which is expected to reach US$237.8 Million by 2030 with a CAGR of a 2.7%. The Central Venous Catheters segment is also set to grow at 4.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $116.6 Million in 2024, and China, forecasted to grow at an impressive 6.5% CAGR to reach $104.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global NICU Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global NICU Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global NICU Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Angiplast Pvt. Ltd, Avanos Medical Inc., Bactiguard AB, B. Braun Melsungen AG, BD (Becton, Dickinson & Co.) and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this NICU Catheters market report include:
- Angiplast Pvt. Ltd
- Avanos Medical Inc.
- Bactiguard AB
- B. Braun Melsungen AG
- BD (Becton, Dickinson & Co.)
- Cook Medical
- Fisher & Paykel Healthcare
- GPC Medical Ltd
- ICU Medical Inc.
- Intersurgical Ltd.
- Kangaroo Medical (Cardinal Health)
- Kindwell Medical
- La-med Healthcare Pvt Ltd
- Medline Industries, LP
- Medtronic plc
- NeoMedical Inc.
- Neotech Products, LLC
- Smiths Medical (ICU Medical)
- Teleflex Incorporated
- Vygon Group
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Angiplast Pvt. Ltd
- Avanos Medical Inc.
- Bactiguard AB
- B. Braun Melsungen AG
- BD (Becton, Dickinson & Co.)
- Cook Medical
- Fisher & Paykel Healthcare
- GPC Medical Ltd
- ICU Medical Inc.
- Intersurgical Ltd.
- Kangaroo Medical (Cardinal Health)
- Kindwell Medical
- La-med Healthcare Pvt Ltd
- Medline Industries, LP
- Medtronic plc
- NeoMedical Inc.
- Neotech Products, LLC
- Smiths Medical (ICU Medical)
- Teleflex Incorporated
- Vygon Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 284 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
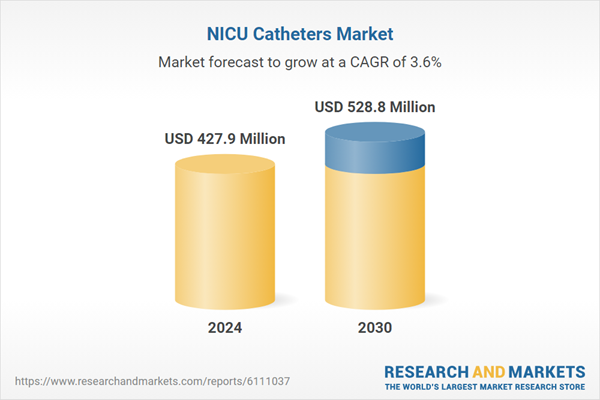
| Estimated Market Value ( USD | $ 427.9 Million |
| Forecasted Market Value ( USD | $ 528.8 Million |
| Compound Annual Growth Rate | 3.6% |
| Regions Covered | Global |