Market Size & Trends
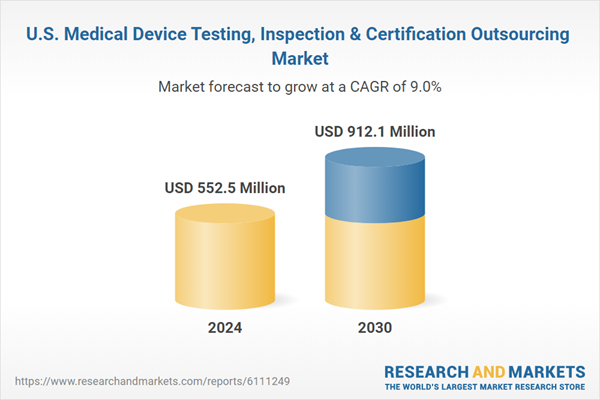
The U.S. medical device testing, inspection & certification outsourcing market size was estimated at USD 552.5 million in 2024 and is projected to grow at a CAGR of 9.0% from 2025 to 2030. The growth is driven by the increasing demand for regulatory compliance, the increasing technological complexity of medical devices, especially those with digital health features, and the growing need for faster time-to-market. These trends are driving device manufacturers to partner with experienced TIC providers to meet safety and quality standards, optimize development cycles, and ensure regulatory alignment across global markets.Rising regulatory complexity in the U.S. medical device industry is prompting a notable shift toward outsourcing of testing, inspection, and certification services. Manufacturers are navigating a landscape shaped by more rigorous FDA guidelines covering cybersecurity protocols, usability engineering, and biocompatibility testing. These demands have made in-house regulatory compliance increasingly resource-intensive, especially for small and mid-sized firms. As a result, medical device companies are turning to specialized TIC providers with deep regulatory knowledge and accredited infrastructure. These firms not only help navigate 510(k) and PMA pathways but also provide documentation and data needed for regulatory submissions. The partnership reduces internal operational burden and improves the accuracy and completeness of testing outputs.
The increasing complexity of medical devices, particularly with the integration of digital and connected technologies, is intensifying demand for specialized validation and performance testing. Several modern devices now include embedded software, wireless modules, AI-powered diagnostics, and sensor technologies that require advanced testing environments and simulation tools. In-house teams often lack the capabilities to validate these features against real-world conditions or emerging safety standards. TIC providers offer tailored services such as software lifecycle testing, electromagnetic compatibility (EMC) assessments, wireless signal reliability testing, and cloud platform security evaluations. These services are becoming essential as the market shifts toward wearable health monitors, remote diagnostic tools, and robotic-assisted surgical systems. The growing reliance on such devices in both clinical and home settings necessitates more comprehensive testing, which many companies are outsourcing to accelerate development. As device functionalities evolve to meet new clinical demands, outsourcing allows manufacturers to keep pace with innovation while maintaining robust safety validation.
U.S. Medical Device Testing, Inspection & Certification Outsourcing Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, the analyst has segmented the U.S. medical device testing, inspection, and certification outsourcing market report based on service, device class, and end use.Service Outlook (Revenue, USD Million, 2018 - 2030)
- Testing
- Inspection
- Certification
Device Class Outlook (Revenue, USD Million, 2018 - 2030)
- Class I
- Class II
- Class III
End Use Outlook (Revenue, USD Million, 2018 - 2030)
- Medical Device Companies
- Pharmaceutical and Biotech Companies
- Others
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned
- SGS SA
- Intertek Group plc
- Eurofins Scientific SE
- DEKRA CERTIFICATION B.V.
- UL Solutions Inc.
- TÜV SÜD
- ALS Limited
- Bureau Veritas SA
- Element Materials Technology
- Nelson Labs
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | June 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 552.5 Million |
| Forecasted Market Value ( USD | $ 912.1 Million |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |