Market Size & Trends
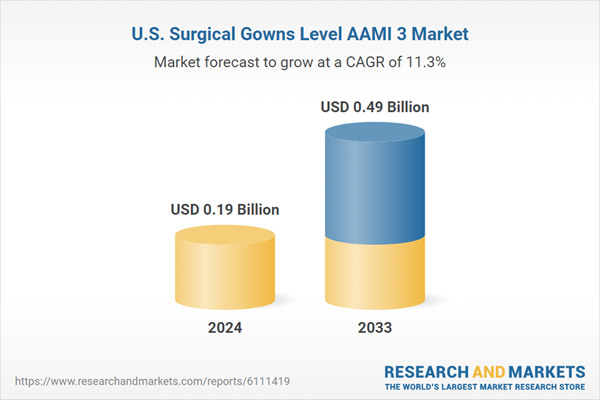
The U.S. surgical gowns level AAMI 3 market size was estimated at USD 0.19 billion in 2024 and is projected to reach USD 0.49 billion by 2033, growing at a CAGR of 11.31% from 2025 to 2033. The market is primarily driven by growing awareness of infection prevention, enhanced safety and infection control regulations, increasing minimally invasive surgical procedures, and technological advancements in gown materials.The U.S. surgical gowns Level AAMI 3 market is driven primarily by the increasing demand for infection prevention in healthcare settings, the rising number of arthroscopic orthopedic, endoscopic urological, and mastectomy procedures in the U.S. For instance, according to the Knee Pain Centers of America article published in February 2025, about 700,000 knee arthroscopies are performed yearly, resulting in costs exceeding USD 3.0 billion annually. Further, according to the University of Florida Health, kidney stone disease impacts roughly 1 in every 500 people in the U.S. annually. Throughout their lifetime, about 1 in 8 men-most commonly between ages 40 and 60, and one in sixteen women-most commonly between ages 20 and 50-will experience this condition. In addition, according to Brigham and Women's Hospital, each year, more than 100,000 women undergo some form of mastectomy. Therefore, the increase in kidney stone diseases, breast cancer cases, and the corresponding rise in mastectomy procedures are driving market growth.
AAMI Level 3 surgical gowns offer moderate barrier protection, making them suitable for procedures with moderate fluid exposure, which is common in hospitals and surgical centers. The rising number of surgeries, particularly outpatient procedures, contributes to the growing need for reliable protective apparel. The increasing adoption of minimally invasive procedures, such as laparoscopic surgeries, further propels market growth. For instance, according to an article by North Kansas City Hospital & Meritas Health, approximately 15 million laparoscopic procedures are performed annually in the U.S., making it one of the most frequently conducted surgeries and offering benefits for numerous medical conditions.
Additionally, the growing aging U.S. population contributes to a higher incidence of chronic diseases requiring surgical intervention. Hospitals and surgical centers increasingly seek cost-effective, high-performance gowns, spurring demand for reusable and eco-friendly options. Market consolidation and strategic partnerships among manufacturers and distributors help streamline supply chains and improve availability. Lastly, federal and state funding for hospital preparedness and infection control programs supports sustained investment in high-quality surgical gowns like those meeting AAMI Level 3 standards. In the U.S., founded in 2002, the Administration for Strategic Preparedness & Response, ASPR’s Hospital Preparedness Program (HPP), aims to ready the healthcare delivery system to address community needs, ensure equitable access to care, and save lives during disasters and emergencies. By Section 319C-2 of the Public Health Service (PHS) Act, the Administration for Strategic Preparedness and Response (ASPR) allocates Hospital Preparedness Program (HPP) cooperative agreement funding to recipients based on a legislatively mandated formula. Furthermore, the CDC's Public Health Emergency Preparedness (PHEP) Cooperative Agreement funds 50 states, four cities, and 8 U.S. territories and freely associated states.
Regulatory emphasis on healthcare worker and patient safety has led to stricter compliance requirements, boosting the adoption of certified gowns. For instance, according to Cardinal Health, protective apparel, including surgical gowns, is regulated by standards and testing protocols that all manufacturers must meet. These standards are established by the Association for the Advancement of Medical Instrumentation (AAMI). Specifically, ANSI/AAMI PB70:2012 defines a classification system (Levels 1 through 4) for protective apparel used in healthcare settings, including surgical gowns. This classification is based on liquid barrier performance assessed through standardized testing methods. The U.S. Food and Drug Administration (FDA) has adopted ANSI/AAMI PB70:2012 as the mandatory standard that all surgical gown manufacturers must follow.
Technological advancements in fabric materials such as SMS (Spunbond-Meltblown-Spunbond) and multilayer laminates enhance protection and comfort, increasing market competitiveness.
In addition, the growing prevalence of cancer is also expected to boost demand during the forecast period. For instance, according to the American Cancer Society report, in the U.S. in 2025, approximately 316,950 new cases of invasive breast cancer are expected to be diagnosed in women, along with about 2,800 cases in men. Additionally, an estimated 59,080 cases of ductal carcinoma in situ (DCIS) will be diagnosed in women. The year is projected to see around 42,680 breast cancer-related deaths, with 42,170 occurring in women and 510 in men.
U.S. Surgical Gowns Level AAMI 3 Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis on the latest industry trends in each of the sub-segments from 2021 to 2033. For the purpose of this study, the analyst has segmented the U.S. surgical gowns level AAMI 3 market report on the basis of usability gown type, surgery, and end use:Usability Outlook (Revenue, USD Million, 2021 - 2033)
- Disposable Gowns
- Reusable Gowns
Gown Type Outlook (Revenue, USD Million, 2021 - 2033)
- Fabric Reinforced
- Non-fabric Reinforced
Surgery Outlook (Revenue, USD Million, 2021 - 2033)
- General Surgery
- Orthopedic Surgery
- Endoscopic Surgery
- Gynecological Surgery
- Mastectomy
- Others
End Use Outlook (Revenue, USD Million, 2021 - 2033)
- Operating Room
- Intensive Care Unit (ICU)
- Emergency Room
- Others
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Cardinal Health.
- Owens & Minor
- Medline Industries, LP
- Henry Schein, Inc.
- Boston Scientific Corporation
- Standard Textile Co., Inc.
- Encompass Group, LLC
- Dynarex Corporation
- LDI Solutions, LLC
- Taromed
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 0.19 Billion |
| Forecasted Market Value ( USD | $ 0.49 Billion |
| Compound Annual Growth Rate | 11.3% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |






![Surgical Gowns and Drapes Market by Product Type, Usage, Material [Nonwoven, Woven], Sterility, Application (Cardiovascular, Laparoscopy, End User - Global Forecast to 2030 - Product Image](http://www.researchandmarkets.com/product_images/12895/12895234_60px_jpg/surgical_gowns_and_drapes_market.jpg)

