U.S. Ultomiris Drug Market Size & Trends
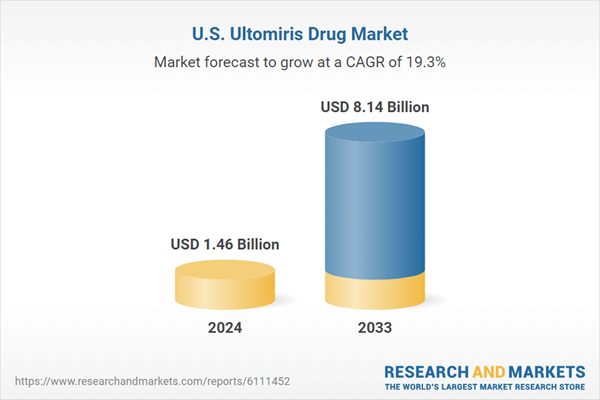
The U.S. ultomiris drug market size was estimated at USD 1.46 billion in 2024 and is projected to reach USD 8.14 billion by 2033, growing at a CAGR of 19.93% from 2025 to 2033. Ultomiris continues to gain traction due to its extended dosing interval, which improves patient compliance and convenience, making it a preferred choice among healthcare providers. In addition, its expanding clinical indications, including generalized myasthenia gravis (gMG) and neuromyelitis optica spectrum disorder (NMOSD), are contributing to market growth.The rising prevalence of rare autoimmune and hematologic conditions, such as PNH, atypical hemolytic uremic syndrome (aHUS), and generalized myasthenia gravis (gMG), fuels market growth. For instance, the FDA’s approval of Ultomiris for adults with anti-AChR antibody-positive gMG in April 2022 has expanded its therapeutic reach, strengthening its position in the neurology segment. In addition, the FDA’s priority review in March 2024 for Ultomiris in neuromyelitis optica spectrum disorder (NMOSD) underscores its robust pipeline. Strategic marketing by AstraZeneca and Alexion, coupled with advanced medical infrastructure and high diagnosis rates in the U.S., supports sustained market expansion. The drug’s role as a long-acting C5 complement inhibitor, administered intravenously every 8 weeks, positions it as a preferred choice for managing complement-mediated disorders.
Ultomiris (ravulizumab-cwvz), developed by Alexion Pharmaceuticals and acquired by AstraZeneca in 2021, has significantly impacted the U.S. rare disease market. In the first half of 2024, Ultomiris generated USD 1.032 billion in U.S. sales, marking a 27% increase over the previous year. This growth is attributed to expanding indications and a shift from its predecessor, Soliris.
In the U.S. hematological disorders market, Ultomiris (ravulizumab), Hemlibra (emicizumab), and Vafseo (vadadustat) collectively contributed 45% of the market’s sales growth in 2023. Ultomiris alone accounted for 21% of this growth, driven by its expanded indications and regulatory approvals.
AstraZeneca's strategic expansion of Ultomiris into neuromyelitis optica spectrum disorder (NMOSD) underscores its commitment to broadening the drug's therapeutic applications. In March 2024, the U.S. FDA approved Ultomiris for NMOSD, making it the first and only long-acting C5 complement inhibitor for this indication. The approval was based on the CHAMPION-NMOSD trial, which demonstrated zero relapses among patients treated with Ultomiris over a median duration of 73 weeks.
U.S. Ultomiris Drug Market Report Segmentation
This report forecasts revenue growth in the U.S. and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, the analyst has segmented the U.S. Ultomiris drug market report based on indication, end use, distribution channel, and region:Indication Outlook (Revenue, USD Million, 2021 - 2033)
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Atypical Hemolytic Uremic Syndrome (aHUS)
- Generalized Myasthenia Gravis (gMG)
- Neuromyelitis Optica Spectrum Disorder (NMOSD)
End Use Outlook (Revenue, USD Million, 2021 - 2033)
- Adult
- Pediatric
Distribution Channel Outlook (Revenue, USD Million, 2021 - 2033)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned
- Alexion Pharmaceuticals (AstraZeneca)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 1.46 Billion |
| Forecasted Market Value ( USD | $ 8.14 Billion |
| Compound Annual Growth Rate | 19.3% |
| Regions Covered | United States |