The spine biologics market represents a rapidly evolving segment within spinal surgery and regenerative medicine, offering biologically derived materials that enhance spinal fusion, support bone healing, and reduce recovery time in spinal procedures. These products include bone grafts (autografts, allografts), bone graft substitutes (demineralized bone matrices, synthetic grafts), cell-based matrices, and growth factors. Spine biologics are increasingly used in spinal fusion surgeries, vertebral reconstruction, and minimally invasive procedures to improve patient outcomes and reduce the risk of complications associated with traditional mechanical implants. The market is fueled by rising incidences of degenerative disc diseases, trauma cases, and spinal deformities - conditions that are especially prevalent in aging populations. Moreover, growing demand for minimally invasive and biologically enhanced procedures is driving research into advanced biologics that accelerate natural bone regeneration and reduce postoperative pain. As the focus in spinal care shifts from structural correction to biological healing and recovery, spine biologics have emerged as a crucial component in next-generation spinal treatment protocols.
In 2024, the spine biologics market witnessed strong growth, supported by a combination of technological advancements and increased procedural volume in both developed and emerging healthcare systems. Surgeons increasingly preferred bioactive and cell-based materials over traditional grafting methods, given their superior integration and potential to stimulate osteogenesis. Biopharmaceutical companies expanded their portfolios with next-generation growth factors and cell therapies, while hospitals adopted pre-filled grafts and customizable delivery systems that reduced operating times. Regulatory agencies began updating guidance frameworks to reflect new clinical data, which enhanced approval pathways for novel biologic products, particularly in the U.S. and Europe. Meanwhile, price sensitivity and limited insurance coverage in several regions influenced purchasing decisions, pushing manufacturers to emphasize value-based performance and long-term fusion success. Collaboration between device manufacturers and biologic developers also intensified, resulting in integrated spinal systems that combine implants with compatible biologic components. As awareness of regenerative spine solutions grew, more patients and providers viewed biologics as essential to modern spine care, especially in complex or revision cases.
Looking ahead to 2025 and beyond, the spine biologics market is poised for continued innovation, driven by personalized medicine, improved clinical data, and expansion into outpatient surgical settings. Next-generation biologics will likely be tailored to individual patient profiles, including genetic and metabolic factors that influence bone healing and graft integration. 3D-bioprinted scaffolds, stem cell-based products, and off-the-shelf allogenic solutions are expected to gain regulatory momentum and wider clinical adoption. In addition, demand for biologics in minimally invasive spine procedures will rise, prompting the development of injectable biologics and delivery systems that are compatible with tubular and endoscopic access. Emerging markets will see stronger uptake of synthetic graft substitutes as governments invest in affordable spinal care infrastructure. Reimbursement models will evolve to reward biologics that demonstrate measurable outcomes such as shorter healing times and lower revision rates. As clinical trials continue to validate the long-term efficacy of biologics over traditional approaches, the market will mature into a cornerstone of regenerative spinal therapy, supporting both fusion and motion-preservation procedures.
Key Insights: Spine Biologics Market
- Surge in demand for cell-based and stem cell-enhanced biologics is reshaping spine fusion procedures by improving healing rates and reducing dependency on autografts and allografts.
- Development of minimally invasive biologic delivery systems is enabling easier application of regenerative materials during endoscopic or tubular spine surgeries, improving efficiency and outcomes.
- Growth in synthetic bone graft substitutes is accelerating due to their scalability, cost-effectiveness, and reduced risk of disease transmission compared to donor-derived materials.
- Advancements in 3D-bioprinting are paving the way for custom-designed biologic scaffolds that mimic natural bone architecture, enhancing integration and spinal stability.
- Integration of biologics with spinal hardware is increasing, with preloaded cages and biologic-friendly implants offering synergistic benefits in spinal reconstruction surgeries.
- Rising global prevalence of spinal disorders, including degenerative disc disease and scoliosis, is creating sustained demand for effective and regenerative spinal treatment solutions.
- Advancements in biotechnology and tissue engineering are expanding the range of available biologics, improving surgical outcomes and reducing complication risks.
- Increased acceptance of outpatient spine surgeries is driving demand for biologics that promote rapid recovery and are compatible with minimally invasive techniques.
- Growing patient and surgeon preference for less invasive, biologically enhanced procedures is encouraging the use of biologics in both initial and revision spinal surgeries.
- High costs and inconsistent reimbursement policies for advanced biologics limit accessibility, particularly in public healthcare systems and emerging markets, where affordability and cost-effectiveness are major purchasing factors.
Spine Biologics Market Segmentation
By Product Type:
- Bone Graft Substitute
- Allograft
- Demineralized Bone Matrix
By Application:
- Spinal Fusion
- Disc Degeneration
- Fracture Repair
By End User:
- Hospitals
- Orthopedic Clinics
- Ambulatory Surgical Centers
By Technology:
- Cell-Based Therapies
- Growth Factor Therapies
By Distribution Channel:
- Direct Sales
- Distributors
By Geography:
- North America (USA, Canada, Mexico)
- Europe (Germany, UK, France, Spain, Italy, Rest of Europe)
- Asia-Pacific (China, India, Japan, Australia, Vietnam, Rest of APAC)
- The Middle East and Africa (Middle East, Africa)
- South and Central America (Brazil, Argentina, Rest of SCA)
Spine Biologics Market Size Data, Trends, Growth Opportunities, and Restraining Factors:
- This comprehensive Spine Biologics market report delivers updated market size estimates from 2024 to 2034, offering in-depth analysis of the latest Spine Biologics market trends, short-term and long-term growth drivers, competitive landscape, and new business opportunities. The report presents growth forecasts across key Spine Biologics types, applications, and major segments, alongside detailed insights into the current Spine Biologics market scenario to support companies in formulating effective market strategies.
- The Spine Biologics market outlook thoroughly examines the impact of ongoing supply chain disruptions and geopolitical issues worldwide. Factors such as trade tariffs, regulatory restrictions, production losses, and the emergence of alternatives or substitutes are carefully considered in the Spine Biologics market size projections. Additionally, the analysis highlights the effects of inflation and correlates past economic downturns with current Spine Biologics market trends, providing actionable intelligence for stakeholders to navigate the evolving Spine Biologics business environment with precision.
Spine Biologics Market Competition, Intelligence, Key Players, and Winning Strategies to 2034:
- The 2025 Spine Biologics Market Research Report identifies winning strategies for companies to register increased sales and improve market share.
- Opinions from senior executives from leading companies in the Spine Biologics market are imbibed thoroughly and the Spine Biologics industry expert predictions on the economic downturn, technological advancements in the Spine Biologics market, and customized strategies specific to a product and geography are mentioned.
- The Spine Biologics market report is a source of comprehensive data and analysis of the industry, helping businesses to make informed decisions and stay ahead of the competition. The Spine Biologics market study assists investors in analyzing On Spine Biologics business prospects by region, key countries, and top companies' information to channel their investments.
- The report provides insights into consumer behavior and preferences, including their buying patterns, brand loyalty, and factors influencing their purchasing decisions. It also includes an analysis of the regulatory environment and its impact on the Spine Biologics industry. Shifting consumer demand despite declining GDP and burgeoning interest rates to control surging inflation is well detailed.
What's Included in the Report?
- Global Spine Biologics market size and growth projections, 2024-2034
- North America Spine Biologics market size and growth forecasts, 2024-2034 (United States, Canada, Mexico)
- Europe market size and growth forecasts, 2024-2034 (Germany, France, United Kingdom, Italy, Spain)
- Asia-Pacific Spine Biologics market size and growth forecasts, 2024-2034 (China, India, Japan, South Korea, Australia)
- Middle East Africa Spine Biologics market size and growth estimate, 2024-2034 (Middle East, Africa)
- South and Central America Spine Biologics market size and growth outlook, 2024-2034 (Brazil, Argentina, Chile)
- Spine Biologics market size, share and CAGR of key products, applications, and other verticals, 2024-2034
- Short- and long-term Spine Biologics market trends, drivers, challenges, and opportunities
- Spine Biologics market insights, Porter’s Five Forces analysis
- Profiles of 5 leading companies in the industry - overview, key strategies, financials, product portfolio and SWOT analysis
- Latest market news and developments
Key Questions Answered in This Report:
- What is the current Spine Biologics market size at global, regional, and country levels?
- What is the market penetration of different types, Applications, processes/technologies, and distribution/sales channels of the Spine Biologics market?
- What will be the impact of economic slowdown/recission on Spine Biologics demand/sales?
- How has the global Spine Biologics market evolved in past years and what will be the future trajectory?
- What is the impact of growing inflation, Russia-Ukraine war on the Spine Biologics market forecast?
- What are the Supply chain challenges for Spine Biologics?
- What are the potential regional Spine Biologics markets to invest in?
- What is the product evolution and high-performing products to focus in the Spine Biologics market?
- What are the key driving factors and opportunities in the industry?
- Who are the key players in Spine Biologics market and what is the degree of competition/Spine Biologics market share?
- What is the market structure /Spine Biologics Market competitive Intelligence?
Available Customizations:
The standard syndicate report is designed to serve the common interests of Spine Biologics Market players across the value chain, and include selective data and analysis from entire research findings as per the scope and price of the publication.However, to precisely match the specific research requirements of individual clients, several customization options are offered to include the data and analysis of interest in the final deliverable.
Some of the customization requests are as mentioned below:
- Segmentation of choice - Clients can seek customization to modify/add a market division for types/applications/end-uses/processes of their choice.
- Spine Biologics Pricing and Margins Across the Supply Chain, Spine Biologics Price Analysis / International Trade Data / Import-Export Analysis.
- Supply Chain Analysis, Supply-Demand Gap Analysis, PESTLE Analysis, Macro-Economic Analysis, and other Spine Biologics market analytics.
- Processing and manufacturing requirements, Patent Analysis, Technology Trends, and Product Innovations.
- Further, the client can seek customization to break down geographies as per their requirements for specific countries/country groups such as South East Asia, Central Asia, Emerging and Developing Asia, Western Europe, Eastern Europe, Benelux, Emerging and Developing Europe, Nordic countries, North Africa, Sub-Saharan Africa, Caribbean, The Middle East and North Africa (MENA), Gulf Cooperation Council (GCC) or any other.
- Capital Requirements, Income Projections, Profit Forecasts, and other parameters to prepare a detailed project report to present to Banks/Investment Agencies.
Additional support:
- All the data presented in tables and charts of the report is provided in a separate Excel document
- Print authentication allowed on purchase of online versions
- 10% free customization to include any specific data/analysis to match the requirement
- 7 days of analyst support
This product will be delivered within 1-3 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | August 2025 |
| Forecast Period | 2025 - 2033 |
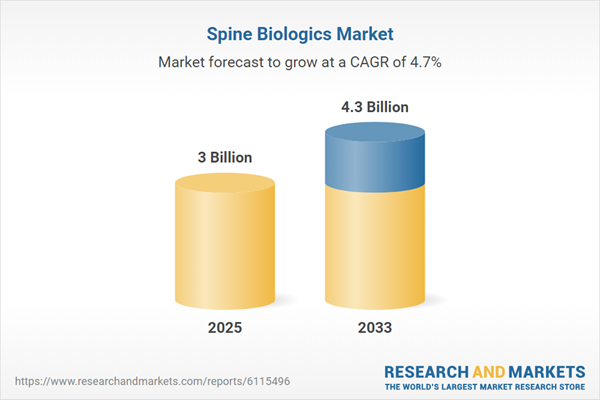
| Estimated Market Value ( USD | $ 3 Billion |
| Forecasted Market Value ( USD | $ 4.3 Billion |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |