Japan Transcatheter Heart Valve Replacement Industry Overview
An aging population and rising rates of aortic valve disorders like aortic stenosis are driving significant growth in Japan's transcatheter heart valve replacement (TAVR) market. Japan, which has one of the longest life expectancies in the world, is dealing with an increasing number of patients who need minimally invasive cardiac procedures. TAVR has become a popular substitute for open heart surgery, especially for high-risk and elderly patients. This increasing tendency is supported by the nation's well-established healthcare system as well as the strong clinical acceptance of cutting-edge cardiovascular technologies. Furthermore, in order to promote quicker market entry and wider access, Japanese regulatory bodies have expedited the approval procedures for novel heart valve devices.One of the main drivers of the TAVR market in Japan is technological advancement. Next-generation transcatheter valves with enhanced deliverability, increased longevity, and fewer difficulties such paravalvular leak or vascular access problems are being actively developed by top local and international manufacturers. Minimally invasive techniques are gaining preference, with improved patient outcomes, shorter hospital stays, and lower post-procedure recovery periods increasing demand. Additionally, hybrid operating rooms are becoming more and more common in Japanese hospitals and cardiac clinics, allowing for safe and effective TAVR treatments. Clinical studies and academic research projects around the country continue to provide evidence supporting wider indications and earlier intervention using TAVR.
The industry still faces a number of obstacles in spite of its expansion. Although it is becoming more flexible, Japan's stringent regulatory framework still needs extensive clinical data before approving new devices. Additionally, even though reimbursement structures are getting better, high procedural and device costs might put a pressure on hospital finances. The scarcity of highly qualified interventional cardiologists and surgeons who are adept at doing TAVR, especially outside of major cities, is another obstacle. However, these shortages are being filled by government assistance, public-private partnerships, and continuing physician training programs. As the Japanese TAVR market develops to accommodate both high - and intermediate-risk patient populations, it is anticipated to experience steady development in the years to come. The industry will have a significant impact on how structural cardiac therapies are developed in Japan in the future thanks to ongoing innovation, strategic alliances, and growing clinical use.
Key Factors Driving the Japan Transcatheter Heart Valve Replacement Market Growth
Increasing Physician Knowledge and Education
The development of physician expertise in transcatheter aortic valve replacement (TAVR) procedures has advanced significantly in Japan. Japanese interventional cardiologists and cardiac surgeons now possess excellent expertise in executing complex structural heart procedures because to specialized training programs that were frequently created in partnership with international cardiovascular centers of excellence. These courses provide a strong emphasis on post-operative care, device handling, imaging interpretation, and patient selection. A growing number of institutions are improving clinical competency through virtual simulations, international fellowships, and hands-on workshops. The wider medical community now has much greater confidence in TAVR as a consequence of the notable improvements in procedural safety and success rates. The treatment is being adopted more quickly as a result of this increasing clinical confidence, which is also helping the TAVR industry in Japan develop overall.Support from Regulations for Innovation
The regulatory landscape in Japan has changed to facilitate the quick uptake of life-saving medical innovations like transcatheter heart valve systems. For novel devices supported by encouraging clinical data, the Pharmaceuticals and Medical Devices Agency (PMDA) and the Ministry of Health, Labour, and Welfare (MHLW) have implemented expedited approval procedures and conditional early approvals. Patients who previously had few options now have more options thanks to the regulatory flexibility that has accelerated the market introduction of next-generation TAVR systems. In order to maintain safety, efficacy, and post-market surveillance standards, regulators are also collaborating closely with healthcare providers and industry stakeholders. Japan's regulatory framework has emerged as a major driver of growth in the transcatheter heart valve replacement business by promoting innovation without sacrificing patient safety.Growing Knowledge and Prompt Diagnosis
Awareness of valvular heart disorders, particularly aortic stenosis, is progressively expanding across Japan due to statewide education initiatives, patient activism, and improved access to diagnostic services. Cardiologists and general practitioners are more watchful when it comes to evaluating older persons for early indications of valve disease utilizing clinical evaluations and echocardiography. Because of this, more patients are receiving diagnoses before their symptoms worsen, which enables prompt referral for less invasive procedures like TAVR. Public awareness of the advantages of early intervention is also being raised by media coverage and community health initiatives. In addition to improving patient outcomes, this move toward proactive diagnosis and care is increasing demand for TAVR procedures and fostering the industry's long-term expansion.Challenges in the Japan Transcatheter Heart Valve Replacement Market
Expensive Procedure and Equipment Expenses
The high expense of the process and the devices is one of the main issues facing the transcatheter heart valve replacement (TAVR) business in Japan. The high cost of heart valve systems and the requirement for specialized infrastructure, like hybrid operating rooms and cutting-edge imaging equipment, make TAVR costly even if it is covered by Japan's national health insurance. Hospital finances may be strained by these expenses, especially in smaller or more regional facilities with tighter budgets. Many rural and community hospitals find it difficult to implement the practice, even though urban hospitals are better able to cover these costs. Despite the procedure's increasing clinical acceptance and advantages for high-risk or elderly patients, this cost-related barrier impedes wider adoption and contributes to unequal access.Limited Skilled Workforce
A highly skilled team comprising interventional cardiologists, cardiac surgeons, imaging specialists, and support personnel is needed to do TAVR. While large-city medical centers in Japan have made great strides in gaining TAVR competence, regional and rural areas continue to lack qualified personnel. The growth of TAVR services outside of urban centers is constrained by this discrepancy. Although international partnerships and training initiatives are aiding in closing the skills gap, it still takes time to become proficient in such intricate processes. Additionally, staffing and succession planning are made more difficult by Japan's aging medical personnel. It may take longer to develop TAVR services to meet rising demand if there isn't an adequate supply of fresh, qualified professionals, particularly outside of cities.Japan Transcatheter Heart Valve Replacement Market Regional Analysis
In cities like Tokyo, Osaka, and Nagoya, where sophisticated cardiac facilities and knowledgeable experts enable greater procedure volumes and quicker acceptance of new technology, the majority of Japan's TAVR industry is focused. The regional analysis is as follows:Tokyo Transcatheter Heart Valve Replacement Market
The market for transcatheter heart valve replacements in Tokyo is growing quickly, supported by the city's top academic and cardiac care institutes. Modern hybrid operating rooms and imaging suites can be found in Tokyo's sophisticated hospitals, allowing for high volumes of TAVR procedures with excellent safety profiles. A proactive local adoption pace is supported by regulatory-clearance partnerships and the early introduction of innovative devices, such as self-expanding and balloon-expandable valves. Procedural increase is being driven by the large number of interventional cardiologists and cardiac surgeons with specific structural heart skills in Tokyo's urban medical workforce. Tokyo is Japan's benchmark region for TAVR innovation, leading the country in technology adoption, case volume, and outcomes research, despite national trends showing a shift toward minimally invasive valve therapies.Kansai Transcatheter Heart Valve Replacement Market
Driven by top medical facilities and university research institutes, the Kansai region, which includes Osaka, Kobe, and Kyoto, is crucial to Japan's transcatheter heart valve replacement (TAVR) business. High procedural volumes with excellent safety results are made possible by the region's major hospitals' hybrid operating rooms and cutting-edge imaging capabilities. Thanks to active partnerships with both domestic and foreign device developers, Kansai has developed into a clinical center for the introduction and assessment of new-generation balloon-expandable and self-expanding valves. Through regional training programs and case-sharing efforts, physician knowledge keeps expanding. Although Kansai's densely populated urban areas and advantageous national insurance reimbursement help the market, regional adoption is still lagging behind Tokyo, suggesting potential for growth in secondary cities and community hospitals.Aichi Transcatheter Heart Valve Replacement Market
Aichi Prefecture is a major center for transcatheter heart valve replacement (TAVR) treatments, and it includes Nagoya, the fourth-largest city in Japan. An increasing number of minimally invasive cardiac procedures are supported by Nagoya's sophisticated medical infrastructure. The area's strong healthcare infrastructure, which includes specialized cardiac facilities and well-equipped hospitals, helps explain why TAVR is becoming more and more popular there. The market is being driven further by the aging population in Aichi Prefecture and the increased awareness of valvular heart disorders, which has increased demand for early diagnosis and treatment. Furthermore, partnerships between regional healthcare facilities and device producers have made it easier to introduce cutting-edge heart valve technologies, improving patient accessibility and procedure results. As a result, Aichi Prefecture is witnessing a consistent growth trajectory in the TAVR market.Market Segmentation
Type
- Transcatheter Aortic Valve Replacement
- Transcatheter Mitral Valve Replacement
- Transcatheter Pulmonary Valve Replacement
Material
- Mechanical valves
- Biological valves
End User
- Hospitals
- Ambulatory Surgical Centers
- Others
Cities
- Tokyo
- Kansai
- Aichi
- Kanagawa
- Saitama
- Hyogo
- Chiba
- Hokkaido
- Fukuoka
- Shizuoka
Company Analysis (Overview, Key Persons, Recent Developments, SWOT Analysis, Revenue Analysis)
- Edwards Lifesciences
- Abbott Laboratories
- Medtronic Plc
- LIVANOVA PLC
- Boston Scientific Corporation
- Artivion, Inc.
- MicroPort Scientific Corporation
- Venus Medtech (Hangzhou) Inc.
Table of Contents
Companies Mentioned
The major companies profiled in this Japan Transcatheter Heart Valve Replacement market report include:- Edwards Lifesciences
- Abbott Laboratories
- Medtronic Plc
- LIVANOVA PLC
- Boston Scientific Corporation
- Artivion, Inc.
- MicroPort Scientific Corporation
- Venus Medtech (Hangzhou) Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | July 2025 |
| Forecast Period | 2024 - 2033 |
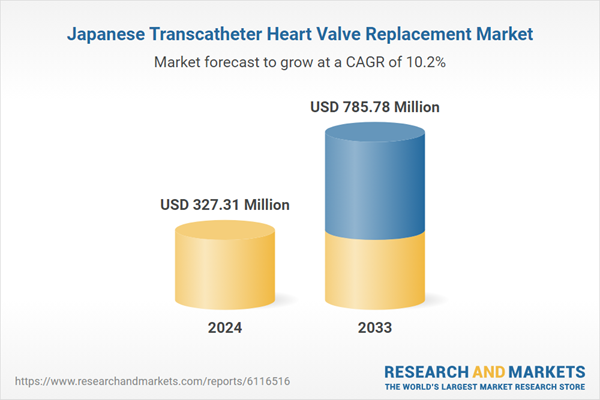
| Estimated Market Value ( USD | $ 327.31 Million |
| Forecasted Market Value ( USD | $ 785.78 Million |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 8 |